Heart Failure: Difference between revisions
No edit summary |
|||
| (86 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
'' | {{TOC limit|1}} | ||
<div align="right"> | |||
'''Ineke Nederend''' MSc, | |||
'''Peter Damman''' MD, | |||
'''W.E.M. Kok''' MD PhD | |||
</div> | |||
Foxglove (digitalis), used as a medicine for heart failure | ==Introduction== | ||
[[File:Foxglove_(digitalis).png|thumb|150px|right|Foxglove (digitalis), used as a medicine for heart failure.]] | |||
== Definition and diagnosis == | ===History=== | ||
{| class="wikitable" border=" | In 1628, William Harvey first described the circulation. Before this time, there was very little understanding of the nature of heart failure (HF). There are, however, accounts of a disease that now would be called heart failure, and herbal medicines such as the ancient boiled bulb of squill, or, later on, the broom plant (Cytisus scoparius) and the foxglove (Digitalis purpura) were used as diuretics to treat heart failure or dropsy (edema). Foxglove was described as a diuretic by William Withering in 1785.<cite>1</cite> The essential glycoside substance digitalis of the leaves of the plant improves contractility of the cardiac muscle and has important parasympathetic effects, particularly on the atrioventricular node. In the 1950s, thiazide diuretics were introduced, and in the 1960s furosemide became available. For a long time, diuretics and digitalis were the main treatment options for HF. Vasodilator therapy for HF was introduced around 1960, and the first randomized trial showing a mortality benefit with nitrates and alphablockers for HF was published in 1986. In 1975, the first ACE inhibitor, captopril, was developed and it was approved for human use in 1981, with data from the first randomized trial being published in 1987. Spironolacton, introduced in 1959, was used (in low dose) for HF only after the introduction of ACE inhibitors. Beta blockers were hardly used in heart failure even though they were shown to beneficial in the 1970s. It was only in 1994 that data from the first randomized trial demonstrated a mortality benefit with beta blocker therapy. | ||
===Framingham heart study=== | |||
In 1948, the Framingham heart study was launched. At its start, 5209 residents of the town Framingham in the USA, aged between 30 and 62 years, were included in the study in an attempt to determine risk factors for cardiac disease. The study is still in progress today and long term data from the lengthy follow up have been published. This study is considered to be the most important longitudinal source of data on the epidemiology of heart failure<cite>2</cite>. | |||
==Definition and diagnosis== | |||
===Definition of heart failure=== | |||
The term heart failure (HF) (congestive heart failure or cardiac decompensation or decompensatio cordis) describes an acute or chronic situation in which the amount of blood pumped through the circulation by the heart, is insufficient to meet the body’s demands at a normal cardiac filling pressure. According to the guidelines of the European Society of Cardiology, HF is defined as a syndrome in which the patient has the following triad of features: (1) symptoms typical of HF; (2) signs typical of HF; and most importantly (3) objective evidence of a structural or functional abnormality of the heart at rest (Table 1). | |||
{| class="wikitable" border="0" cellpadding="0" cellspacing="0" width="600px" | |||
|- | |- | ||
!Table 1. Definition of heart failure | |||
|- | |- | ||
| | |'''''Heart failure is a clinical syndrome in which patients have the following features:''''' | ||
|- | |- | ||
| | | | ||
*'''Symptoms typical of heart failure''' | |||
**Breathlessness | |||
* | **Orthopnoea | ||
**Paroxysmal nocturnal dyspnoea | |||
**Reduced exercise tolerance | |||
**Fatigue | |||
**Tiredness | |||
**Ankle swelling | |||
|- | |- | ||
| | |'''''And''''' | ||
*'''Signs typical of heart failure''' | |||
**Elevated jugular venous pressure | |||
**Hepatomegaly | |||
**Third heart sound | |||
**Pulmonary rales | |||
**Pleura effusion | |||
**Peripheral oedema | |||
**Hepatojugular reflux | |||
**Cardiomegaly on X-ray of the thorax | |||
|- | |- | ||
| | |'''''And''''' | ||
* Objective evidence of a structural or functional abnormality of the heart at rest | *'''Objective evidence of a structural or functional abnormality of the heart at rest''' | ||
**Abnormal echocardiogram | |||
**Abnormal pump function on nuclear imaging or on MRI | |||
|} | |} | ||
=== | ===Prevalence=== | ||
The prevalence of HF in the Western world is estimated to be 1-2%, and the incidence is approximately 5-10 per 1000 persons per year (7,9). Coronary heart disease at a young age is more prevalent in men than women, and so the prevalence of HF is also higher in this group compared to age matched women. In older age groups, the prevalence of HF is equal between the sexes. | |||
=== | ===Acute and chronic heart failure=== | ||
Heart failure | Heart failure may become a chronic condition, in which HF is persistent either with recurrences or with slow progression. A patient may be described as decompensated when chronic stable HF deteriorates. Acute HF has traditionally been used to describe the nature of the clinical presentation, as severe or of recent onset. Different clinical presentations fall under this definition. | ||
=== | ===Systolic versus diastolic heart failure=== | ||
Heart failure patients may be broadly classified into one of two groups, or a combination of both, depending on the left ventricular ejection fraction (LVEF). The LVEF is most often assessed with echocardiography (see Table 3). When the LVEF is less than 45%, systolic pump function is abnormal and it is named systolic HF.<cite>3</cite> If LVEF is preserved (>50%), symptoms are attributed to impaired relaxation of the heart during diastole and therefore this condition is diagnosed as diastolic HF or HF with a preserved LVEF.<cite>3</cite><cite>4</cite> As a result of impaired relaxation, end diastolic pressure and, subsequently, left atrial- and pulmonary pressure will rise; alveolar pulmonary edema develops as a consequence of these changes. LF diastolic dysfunction may be present in asymptomatic patients, and it is considered an important precursor of heart failure.<cite>5</cite> Frequently, patients have both systolic and diastolic heart failure at the same time, but the term for this ailment is still systolic heart failure. | |||
The term heart failure is not limited to a failing left ventricle; the right ventricle may also be involved in the process and there may also be isolated right ventricular heart failure. | |||
=== | ===Pathophysiology of heart failure=== | ||
[[Image:frank_starling.svg|thumb|right|400px|'''Figure 1.''' Frank-Starling curve]] | |||
[[Image:pressure_volume_curve.svg|thumb|400px|'''Figure 2.''' Effects of decreased afterload. Red arrows indicate aortic valve opening, which occurs later and at higher LV systolic pressure when the diastolic aortic pressure is higher. Blue arrows indicate closing of the aortic valve. Bidirectional arrows represent stroke volume. When aortic pressure is decreased, stroke volume increases as a result of a lower aortic pressure during closure of the aortic valve.]] | |||
[[Image:management_outline.svg|thumb|400px|'''Figure 3.''' Management in heart failure.]] | |||
HF is caused by a loss of cardiac pump function, which can be due to a structural abnormality of the heart muscle (e.g. myocardial infarction) or a change in the heart function (and often structure) in response to an abnormal load (e.g. aortic valve stenosis). The relationship between loading the ventricle (by filling it) and its output was described by Frank and Starling in 1918 and has become the cornerstone in understanding heart failure and how to treat it. The relationship states that as a result of loading the heart (increasing its filling or its pressure), the output increases (Figure 1). A heart that has a lower output can be improved by increasing its volume and its loading pressure. This is what naturally happens (LV dilatation and increased filling pressure) when the heart does not pump out enough volume, and, in the first phase of disease, compensates for the loss of contractility. It takes more energy from the heart to work at an increased loading, but the heart has a reasonable energy reserve. In a chronic situation, remodeling of the heart progresses (by hypertrophy of the myocytes and dilatation by increasing myocyte length and matrix changes), which, in the long term, leads to a further loss in function. The result of this dysfunction is further increased loading pressures in the heart and, by communicating the diastolic loading pressures to the left atrium and pulmonary veins, the pulmonary capillaries may become overloaded and leak water into the lungs. This is the practical restriction of further filling the heart as a tool to improve its function; even poor left ventricles may be filled more to increase their output <cite>6</cite> but the patients’ pulmonary capillaries cannot tolerate these hydrostatic pressures and start to leak water. | |||
Hemodynamic explanations (the heart as a pump) use the concept of preload (filling) and afterload (workload of the heart, which is wall tension and arterial pressure or vascular resistance). In this way, the progression of left sided heart failure towards right sided heart failure is explained as follows: prolonged left ventricular failure increases pressures in the left atrium (preload), which in time leads to a subsequent increased resistance in the pulmonary vascular system (which is the afterload of the right ventricle) and eventually may also lead to right ventricular failure. Another relevant issue is afterload of the left ventricle influencing the output of the heart: as the afterload of the aortic pressure also influences the timing of closure of the aortic valve, a high aortic pressure will close the aortic valve early and will, therefore, diminish the output. Decreasing (theoretically diastolic, but more practically systolic) aortic pressure will increase the stroke volume by latter closure of the aortic valves. (Figure 2) | |||
Hormonal/ sympathetic system mechanisms (RAAS/ Sympathetic overstimulation) of heart failure are as important as the hemodynamic mechanisms of heart failure. | |||
A decreased cardiac output leads to diminished renal perfusion and release of hormones in the RAA-system: renin is released into the circulation by the renal juxtaglomerular apparatus, which stimulates the cleavage of angiotensinogen into angiotensin I and II during its passage through the lungs. Angiotensin II stimulates vasoconstriction in the kidneys, and in other vascular systems, increasing blood pressure; the second effect of angiotensin is to stimulate the release of aldosterone from the adrenals into the plasma, which retains sodium from the kidney tubules in the blood and thereby water. The RAA –system, which works as a compensatory mechanism for heart failure to increase blood pressure and blood volume, also stimulates hypertrophy of muscle cells and the formation of fibrosis, which in the long term are detrimental to heart failure. | |||
The other compensatory mechanism for heart failure, stimulation of the sympathetic nervous system, increases heart rate to increase cardiac output, which is a powerful compensatory mechanism. However, chronic stimulation of the sympathetic nerves to the heart, leading to higher heart rates, is toxic to the heart, because of continuous release of norepinephrine to the myocyte. In addition, as a result of their continued stimulation, the betareceptors for norepinephrine are downregulated in heart failure, which further diminishes the function and functional reserve of the heart. | |||
=== | ===Management=== | ||
When a patient presents with symptoms of heart failure, it is worthwhile to have a dedicated diagnostic and therapeutic plan, in the order as indicated below (Figure 3). Clinical aspects are important for diagnosis, but the final diagnosis is only made after objective evidence of heart dysfunction. | |||
===== | ==Clinical aspects== | ||
===History=== | |||
A careful history of the patient is important for the diagnosis and in order to identify the cause of HF. The history (and physical examination) can be used to differentiate between the abovementioned potential causes of HF (refer to Etiology of heart failure). Family history of HF, smoking status, hyperlipidaemia, hypertension and diabetes mellitus are factors that should be taken into account during the assessment of the patient history in order to draw a risk profile of the patient. Finally, the history should include previous events and the response to therapy. | |||
=== | ===Symptoms and signs=== | ||
HF can manifest with a multitude of different symptoms and signs, but shortness of breath and tiredness are the most characteristic. The Framingham Heart Study defined major and minor diagnostic criteria for HF. | |||
'''Major criteria:''' | |||
*Paroxysmal nocturnal dyspnea | |||
*Neck vein distention | |||
*Pulmonaty rales | |||
*Radiographic cardiomegaly (increasing heart size on chest radiography) | |||
*Acute pulmonary edema | |||
*S3 gallop | |||
*Increased central venous pressure (>16 cm H2O at right atrium) | |||
*Hepatojugular reflux | |||
*Weight loss >4.5 kg in 5 days in response to treatment | |||
'''Minor criteria:''' | |||
*Bilateral ankle edema | |||
*Nocturnal cough | |||
*Dyspnea on ordinary exertion | |||
*Hepatomegaly | |||
*Pleural effusion | |||
*Tachycardia (heart rate>120 beats/min.) | |||
Minor criteria are acceptable only if they cannot be attributed to another medical condition (such as pulmonary hypertension, chronic lung disease, cirrhosis, ascites, or the nephrotic syndrome). | |||
Diagnosis of HF requires the simultaneous presence of at least 2 major criteria or 1 major criterion in conjunction with 2 minor criteria. The Framingham Heart Study criteria are 100% sensitive and 78% specific for identifying persons with definite congestive heart failure in an outpatient population.<cite>10</cite> | |||
=== | ===Severity of HF=== | ||
In general, correlation between the severity of symptoms and the severity of HF in terms of loss of maximal oxygen consumption is weak.<cite>3</cite> The New York Heart Association functional classification is used most frequently to classify the severity of HF (Table 2). Assessing severity is needed for the proper therapy/ medication to be chosen. | |||
{| class="wikitable" border="0" cellspacing="0" cellpadding="0" width="600px" | |||
{| class="wikitable" border=" | |||
|- | |- | ||
!colspan="2"|Table 2. NYHA functional classification | |||
|- | |- | ||
| colspan="2 | |colspan="2"|'''''Severity based on symptoms and physical activity''''' | ||
|- | |- | ||
| width=" | |width="20%"|'''Class I''' | ||
|No limitation of physical activity. | |||
Ordinary physical activity does not cause undue fatigue, palpitation, or dyspnoea. | |||
|- | |- | ||
|'''Class II''' | |||
|Slight limitation of physical activity. | |||
Comfortable at rest, but ordinary physical activity results in fatigue, palpitation, or dyspnoea. | |||
|- | |- | ||
|'''Class III''' | |||
|Marked limitation of physical activity. | |||
|- | |||
Comfortable at rest, but less than ordinary activity results in fatigue, palpitation, or dyspnoea. | |||
|- | |||
|'''Class IV''' | |||
|Unable to carry on any physical activity without discomfort. | |||
Symptoms at rest. | |||
If any physical activity is undertaken, discomfort is increased. | |||
|} | |} | ||
===Physical examination=== | |||
There are several key features in the clinical examination of a patient presenting with HF | There are several key features in the clinical examination of a patient presenting with HF. The physical examination should focus on the general appearance of the patient, pulse and blood pressure, signs of fluid overload (increased jugular venous pressure, peripheral edema, ascites and hepatomegaly), the lungs, and the heart (apex, Gallop rhythm, third heart sound, murmurs). | ||
===Additional diagnostic tests=== | |||
In order to assist in the diagnosis of HF and to differentiate between possible causes of HF, the following tests are available. | |||
=== | ===Electrocardiogram=== | ||
An electrocardiogram (ECG) should be performed on every patient suspected of HF. Several common abnormalities (including possible causes) indicative of HF on the ECG include but are not limited to: sinus tachy- or bradycardia, atrial tachycardia, -flutter, or –fibrillation, ventricular arrhythmias, ischemia (including myocardial infarction), abnormal Q waves, left ventricular hypertrophy, micro voltages, and QRS length >120 ms. Although an abnormal ECG (excluding arrhythmias) has a low positive predictive value for HF, a normal ECG is highly indicative of the absence of HF. | |||
=== | ===Chest X-ray=== | ||
A chest X-ray is a part of the standard examination in potential HF patients. Importantly, the X-ray is a tool to detect cardiomegaly (defined as a cardiac: thoracic ratio of > 0,5) or other clues (redistribution, Kerley B-lines and pleural effusion) that indicate HF. It is also important to rule out other causes of dyspnea. | |||
=== | ===Echocardiography=== | ||
Echocardiography is the cornerstone in the diagnosis of HF, and should be performed routinely, because ventricular function can be evaluated accurately with this technique. It can provide objective evidence of a structural or functional abnormality of the heart at rest, besides signs and symptoms that are typical of heart failure. Important parameters that can be assessed include, but are not limited to, wall motion, valve function, left ventricular ejection fraction and diastolic function. Diastolic dysfunction might be an important finding in symptomatic patients with a preserved ejection fraction. Refer to Table 3 for common echocardiographic findings in HF. Transesophageal echocardiography is indicated in patients with an inadequate transthoracic echo window, suspected endocarditis, complicated valvular disease or to exclude a LV thrombus. If echocardiography provides inadequate information or in patients with suspected coronary artery disease, additional imaging includes CT scanning, cardiac magnetic resonance imaging or radionuclide imaging. | |||
=== | ===Laboratory tests=== | ||
[[Image:suspected_heart_failure.svg|thumb|400px|'''Figure 4.''' Flowchart suspected heart failure <cite>3</cite>]] | |||
A standard blood assessment includes a complete blood count, electrolytes, renal function, glucose and liver function. Furthermore, urinalysis and other tests, depending on the clinical condition of the patient, complete the laboratory assessment. For example, cardiac troponins must be sampled if an ACS is in the differential diagnosis. In patients suspected of HF, values of natriuretic peptides (such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP)) can provide important information regarding the diagnosis, management and prognosis of HF. Natriuretic peptides are enzymes, secreted by the atria or ventricles in response to myocardial wall stress. The most commonly used tests are BNP and NT-proBNP measurements, which despite their different half-lives in the plasma, do not differ substantially in terms of diagnostic ability. Cut-off values are different in acute settings with acute dyspnea compared to chronic settings. Normal values are almost 100% specific, and exclude heart failure in patients >18 year old. Abnormal values do not have a 100% predictive value, and objective evidence for heart failure is still needed. The values for BNP and NTproBNP are also used to evaluate the prognosis in patients with known HF, in whom higher values carry a worse prognosis. | |||
A standard blood assessment | |||
===Exercise test=== | |||
This test is | An exercise test is not diagnostic for HF, but may be used to identify ischemia as the cause of heart failure, or it can be used to assess the severity of HF, usually in conjunction with maximal oxygen uptake (VO<sub>2max</sub>) measurement. This test is performed on a treadmill or on a bicycle ergo meter. The patient is asked to give maximal effort while the workload gradually increases. During the test, the ECG is monitored for ischemia. When possible, oxygen consumption should also be measured during the test. Not only is an oxygen consumption test a good tool to discriminate between lung- peripheral- or heart problems, but the obtained value for maximal oxygen uptake (VO<sub>2max</sub>) has an important prognostic value. | ||
===Heart catheterization=== | |||
Heart catheterization is not part of the routine diagnosis and work-up of patients with HF. | Heart catheterization is not always part of the routine diagnosis and work-up of patients with HF. It should be considered however to exclude coronary heart disease (Class of recommendation IIa, level of evidence C, see [[Heart_Failure_Table_4|Table 4]]). Coronary angiography is recommended in patients at high risk of coronary artery disease (Class of recommendation I, level of evidence C) and in HF patients with significant valvular disease (Class of recommendation IIa, level of evidence C). | ||
Table 3 Common echocardiographic abnormalities in heart failure | {| class="wikitable" border="0" cellpadding="0" cellspacing="0" width="650px" | ||
Measurement Abnormality Clinical implications | |- | ||
LVEF Reduced (< | !colspan="3"|Table 3. Common echocardiographic abnormalities in heart failure | ||
|- | |||
!Measurement | |||
!Abnormality | |||
!Clinical implications | |||
|- | |||
|Left ventricular ejection fraction (LVEF) | |||
|Reduced (< 50%) | |||
|Left ventricular global systolic dysfunction | |||
|- | |||
|Left ventricular wall motion | |||
|Akinesis, hypokinesis, dyskinesis | |||
|Myocardial infarction/ischaemia, | |||
Cardiomyopathy, | |||
Myocarditis | |||
|- | |||
|Left ventricular end-diastolic diameter | |||
|Increased (≥60 mm/>32 mm/m2)) | |||
|Volume overload HF likely | |||
|- | |||
|Left ventricular end-systolic diameter | |||
|Increased (≥45 mm/>25 mm/m2,) | |||
|Volume overload | |||
HF likely | HF likely | ||
|- | |||
|Left ventricular fractional shortening | |||
|Reduced (<25%) | |||
Left atrial | |Left ventricular systolic dysfunction | ||
Left ventricular thickness Hypertrophy (>11 – 12 mm) Hypertention, | |- | ||
Valvular structure and function Valvular stenosis or regurgitation (especially aortic stenosis and mitral insufficiency) May be primary cause of HF or complicating factor | |Left atrial volume index | ||
|Increased (volume >34 mL/m2) | |||
|Increased filling pressures, | |||
Mitral valve dysfunction | |||
|- | |||
|Left ventricular thickness | |||
|Hypertrophy (>11 – 12 mm) | |||
|Hypertention, | |||
Aortic stenosis, | |||
Hypertrophic cardiomyopathy | |||
|- | |||
|Valvular structure and function | |||
|Valvular stenosis or regurgitation (especially aortic stenosis and mitral insufficiency) | |||
|May be primary cause of HF or complicating factor | |||
Asses haemodynamic consequences | Asses haemodynamic consequences | ||
Consider surgery | Consider surgery | ||
Mitral diastolic flow profile Abnormalities of the early and late diastolic filling patterns Indicates diastolic dysfunction and suggests mechanism | |- | ||
Tricuspid regurgitation peak velocity Increased (>3 m/s) Increased right ventricular systolic pressure | |Mitral diastolic flow profile | ||
|Abnormalities of the early and late diastolic filling patterns | |||
Pericardium Effusion, | |Indicates diastolic dysfunction and suggests mechanism | ||
|- | |||
|Tricuspid regurgitation peak velocity | |||
|Increased (>3.4 m/s) | |||
|Increased right ventricular systolic pressure | |||
|- | |||
|Pericardium | |||
|Effusion, | |||
Haemopericardium, | |||
Calcification | |||
|Consider tamponade, | |||
Malignancy, | |||
Systemic disease, | |||
Acute or chronic pericarditis, | |||
Constrictive pericarditis | |||
|- | |||
|Aortic outflow velocity time integral | |||
|Reduced (<15 cm) | |||
|Reduced low stroke volume | |||
|- | |||
|Right ventricular function (e.g. TAPSE) | |||
|Reduced (TAPSE < 16 mm) | |||
|RV systolic dysfunction | |||
|- | |||
|Inferior vena cava | |||
|Dilated, with no respiratory collapse | |||
|Increased right atrial pressures, | |||
Right ventricular dysfunction, | |||
=== | Volume overload Pulmonary hypertention possible | ||
The | |} | ||
==Etiology of heart failure== | |||
===Coronary heart disease=== | |||
The most important cause (50% of the cases) of HF in the Western world is ischemic heart disease, including myocardial infarction. These patients mainly suffer from systolic HF due to wall motion abnormalities of the affected area and re-modeling of the non-affected parts of the myocardium. | |||
===Hypertension=== | |||
In patients with a high systolic blood pressure (BP), the left ventricle faces an increased afterload (a higher workload pumping the blood against the increased vascular resistance). Over a certain period of time, this will lead to hypertrophy of the cardiac myocardium, and longer term remodeling may lead to pump function disorders (diastolic or systolic). In as many as 60-70% of patients suffering HF, hypertension is the primary or secondary cause of the condition. | |||
===Heart rhythm disorders=== | |||
Atrial fibrillation is a common rhythm disorder in the elderly. With this condition, the atria do not contract in the coordinated fashion as they would in normal sinus rhythm, and therefore the atria never optimally empty. Normally, the ‘atrial kick’ contributes approximately 15% of the stroke volume. The absence of the atrial kick during atrial fibrillation can contribute to a reduced LVEF. However, atrial fibrillation is seldom the cause of heart failure, but more often a trigger of heart failure in already existing structural heart disease. | |||
===Valvular disease=== | |||
Valvular disease, especially mitral- or aortic, can cause volume and pressure overload of the left ventricle of the heart. This overload causes dilation and / or hypertrophy of the left ventricle, which in the long term decreases the pump function. | |||
===Cardiomyopathies=== | |||
Dilated cardiomyopathy (DCM) is characterized by dilatation of one or both of the ventricles of the heart, with a general decrease in contractility and consequently a decreased pump function. In approximately 30% of the cases, DCM is hereditary. | |||
Hypertrophic cardiomyopathy (see also Hypertension) is characterized by hypertrophy, which may be concentric or asymmetric. The asymmetric form is usually hereditary. | |||
Restrictive cardiomyopathy is characterized by a primary diastolic dysfunction of one or more of the ventricles, leading to increased filling pressures and hypertrophy, and initially a preserved systolic function. | |||
Arrhythmogenic right ventricular cardiomyopathy is characterized by fatty infiltration and fibrosis of the right ventricle or the left ventricle or both and is usually hereditary. | |||
===Pericardial disease and Tamponade=== | |||
Restriction of ventricular filling by a tight (inflamed or constrictive) pericardium or by pericardial effusion and tamponade can be the cause of diastolic HF. | |||
===Drugs=== | |||
*'''Drugs that can cause HF are:''' | |||
**Cytotoxic agents (chemotherapy, especially doxorubicin) | |||
**The antipsychotic agent clozapine | |||
*'''Drugs that can aggravate HF are:''' | |||
**Beta blockers | |||
**Calcium antagonist | |||
**Antiarrhythmics | |||
**Disulfiram | |||
===Toxins=== | |||
*Alcohol | |||
*Cocaine, | |||
*Trace elements (mercury, cobalt, arsenic). | |||
===Endocrine disorders=== | |||
*Diabetes mellitus | |||
*Hypo- or hyperthyroidism | |||
*Cushing syndrome | |||
*Adrenal insufficiency | |||
*Excessive growth hormone in acromegaly | |||
*Phaeochromocytoma | |||
===Nutritional status=== | |||
Deficiency of thiamine, selenium, or camitine, in states of severe cachexia. | |||
===Infiltrative and storage disorders=== | |||
*[[Sarcoidosis]] | |||
*[[Amyloidosis]] | |||
*[[Haemochromatosis]] | |||
*Connective tissue disease | |||
===Infectious disease=== | |||
*[[Chagas’ disease]] | |||
*[[HIV infection]] | |||
*Viral, bacterial or protozoal diseases causing myocarditis. | |||
==Management in investigating etiology of heart failure== | |||
#Assess globally: are there triggers of heart failure. Hypertension, infection, anemia, rhythm disorders. Perform standard laboratory tests: in addition to hemoglobin, leukocytes, thrombocytes, creatinin, sodium and potassium levels, also liver function, thyroid function (TSH), glucose. | |||
#Assess ischemia: are there indications of ischemic etiology (ECG: Q’s or significant and changing ST segments, laboratory: troponins, and echocardiogram: segmental wall motion abnormality in coronary territory areas) ? If yes, then proceed with further coronary artery or myocardial perfusion imaging. | |||
#Are there no indications for ischemic etiology? | |||
#*Classify phenotype of cardiomyopathy: dilated, hypertrophic, restrictive, arrhytmogenic right ventricular cardiomyopathy. | |||
#*Then assess with additional laboratory tests, including creatinin kinase, autoimmune markers, eosinophilia, ferritin and iron saturation. In some suspected cases: calcium and albumin. | |||
#*Look for clues of etiologies: history, family history (including maternal inheritage of diabetes in a family) | |||
#*In case of fever look for infectious etiology, MRI confirmation for possible myocarditis, plasma serology. | |||
#*Look for clues on ECG: microvoltage on the ECG, AV block in combination with later atrial fibrillation. Additional lab may be warranted (monoclonal proteins in case of microvoltage in the presence of sufficient amounts of myocardium and the absence of pericardial fluid or pulmonary emphysema) | |||
#*MRI for further classification of cardiomyopathy and assessment of presence and localization of delayed contrast enhancement | |||
#*Coronary arteriography or coronary CT scan to exclude coronary artery disease | |||
#*Myocardial biopsy in cases where the suspicion of severe underlying disease is high (e.g. fulminant myocarditis, sarcoidosis suspicion on MRI with no other organ involved). | |||
#*Genetic testing after counseling | |||
==Therapy of heart failure== | |||
The therapeutic management of HF involves both pharmacological and non-pharmacological treatment. The goal is reduction in mortality and morbidity, prevention of the progression of HF, and the treatment of (non-) cardiovascular co-morbidities. | |||
===Non-pharmacological treatment=== | |||
Non-pharmacological management is of great importance for HF patients. It can have a significant impact on symptoms, functional capacity, wellbeing, morbidity, and prognosis. The most important non-pharmacological options are described below. | |||
===Education=== | |||
Education of both the patient and their family about HF and its symptoms is important. The patient and/or the caregiver should be able to undertake appropriate actions such as adjusting the diuretic dose or contact the physician when necessary. (Class I recommendation, level of evidence C; see Table 4) Education on the importance and (side) effects of medication should be provided to the patient in order to increase compliance. (Class I recommendation, level of evidence C) | |||
===Fluid and sodium restriction=== | |||
In patients with severe symptoms of HF, restriction of fluid intake (to 1500 ml/day) may be considered. (Class IIa recommendation, level of evidence C). Also, patients should be educated about the salt content of food and advised to minimize their salt intake (< 2 gram/ day) in order to prevent fluid retention. (Class I recommendation, level of evidence C) | |||
===Body weight=== | |||
CHF patients should carefully monitor their body weight. A sudden increase in weight is a potential consequence of fluid retention and deterioration of HF. If patients notice a weight gain of >2kg in 3 days, they should consult their physician. (Class I recommendation, level of evidence C). In obese patients (body mass index >30 kg/m<sup>2</sup>), weight reduction should be promoted to prevent progression of HF, decrease symptoms and improve the overall wellbeing of the patient. (Class IIa recommendation, level of evidence C). Also, attention should be paid to weight loss due to malnutrition, which is frequently observed in severe HF. An altered metabolism, inflammatory mechanisms or a decreased food intake may be important factors in the pathophysiology of cardiac cachexia in HF. (Class I recommendation, level of evidence C) | |||
===Alcohol and tobacco=== | |||
Alcohol intake should be minimized, as it may increase blood pressure and/or have a negative inotropic effect. (Class IIa recommendation, level of evidence C). Smoking cessation should be encouraged. It is recommended that patients with HF receive support and advice on this topic. (Class I recommendation, level of evidence C). A reduction in alcohol and tobacco intake might also improve co-morbidities, including sleep disorders. | |||
===Exercise=== | |||
Exercise training is recommended to all chronic stable HF patients. Twenty years ago, exercise was strongly discouraged in patients with HF as it was considered to be harmful. Nowadays, numerous studies have demonstrated the opposite. Rehabilitation programs have shown to increase exercise capacity and health related quality of life, and decrease hospitalization rates and symptoms. (Class I recommendation, level of evidence A) | |||
===Other=== | |||
Other non-pharmacological treatment recommendations include immunization of HF patients (pneumococcal- and influenza vaccination should be considered), the consulting of a physician around pregnancy, the screening for depression and sleep disorders that require additional medical attention. | |||
==Pharmacological treatment== | |||
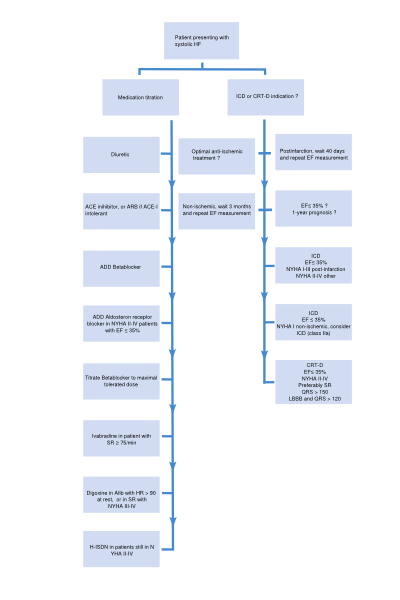
A flowchart for the treatment of patients presenting with systolic HF is depicted in Figure 5. Medications with a class I indication in patients with systolic heart failure are summarized in Table 5. Indications, mode of action, contraindications of the medication, and possible side effects of drugs included in this algorithm are discussed below. | |||
[[Image:management_chronic_systolic_hf.svg|thumb|400px|'''Figure 5.''' Treatment options for patients with chronic systolic HF]] | |||
{| class="wikitable" border="1" cellpadding="1" cellspacing="1" width="600px" | |||
|- | |||
|bgcolor="1E90FF"| | |||
|bgcolor="1E90FF" align="center"|'''NYHA I & EF <40%''' | |||
|bgcolor="1E90FF" align="center"|'''NYHA II''' | |||
|bgcolor="1E90FF" align="center"|'''NYHA III''' | |||
|bgcolor="1E90FF" align="center"|'''NYHA IV''' | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''Diuretic''' | |||
|bgcolor="99FFCC"| | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''ACE-inhib''' | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''AT-II antagonist''' | |||
|bgcolor="99FFCC"| | |||
|bgcolor="9ACD32" align="center"|'''Alternative''' | |||
|bgcolor="9ACD32" align="center"|'''Alternative''' | |||
|bgcolor="9ACD32" align="center"|'''Alternative''' | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''Betablocker''' | |||
|bgcolor="9ACD32" align="center"|'''Post infarction''' | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''Aldosteron antagonist''' | |||
|bgcolor="99FFCC"| | |||
|bgcolor="9ACD32" align="center"|'''EF < 35%''' | |||
|bgcolor="9ACD32" align="center"|'''EF < 35%''' | |||
|bgcolor="9ACD32" align="center"|'''EF < 35%''' | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''Nitrate / Hydralazine''' | |||
|bgcolor="99FFCC"| | |||
|bgcolor="9ACD32" align="center"|'''Afro-American''' | |||
|bgcolor="9ACD32" align="center"|'''Afro-American''' | |||
|bgcolor="9ACD32" align="center"|'''Afro-American''' | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''Ivabradine''' | |||
|bgcolor="99FFCC"| | |||
|bgcolor="9ACD32" align="center"|'''SR>75/min & EF<35%''' | |||
|bgcolor="9ACD32" align="center"|'''SR>75/min & EF<35%''' | |||
|bgcolor="9ACD32" align="center"|'''SR>75/min & EF<35%''' | |||
|- | |||
|bgcolor="99FFCC" align="center"|'''Digoxin''' | |||
|bgcolor="99FFCC"| | |||
|bgcolor="99FFCC"| | |||
|bgcolor="9ACD32"| | |||
|bgcolor="9ACD32"| | |||
|- | |||
|colspan="5"|'''Table 5.''' Medication with a class I indication in patients with systolic heart failure. Note that AT-II antagonists are alternative medicine for ACE inhibitors in case of intolerance (coughing, allergy). Nitrates and Hydralazine are added therapy for patients of Afro-American descent, and alternative therapy for patients that cannot tolerate ACE-inhibitors and AT-II antagonists). Digoxin can also be seen as symptomatic (instead of added preventive) treatment, not always necessary in NYHA III or even IV. | |||
|} | |||
Angiotensin-converting enzyme (ACE) inhibitors | ===Angiotensin-converting enzyme (ACE) inhibitors=== | ||
ACE | An ACE inhibitor is indicated for every patient with symptomatic systolic HF and an EF ≤40 % (NYHA class II-IV). (Class I recommendation, level of evidence A) Contraindications for the use of ACE inhibitors are: | ||
*History of angioedema | |||
*Bilateral renal artery stenosis | |||
*Serum potassium concentration >5.0 mmol/L | |||
*Serum creatinine >220 µmol/L | |||
*Severe aortic stenosis | |||
ACE inhibitors relieve the heart by decreasing the preload and afterload. This is achieved through two mechanisms. Firstly, conversion of angiotensin-I to angiotensin– II is inhibited, which reduces vasoconstriction and lowers BP. Secondly, production of aldosterone is decreased, as angiotensin II induces this production. Aldosterone stimulates sodium- and water retention. Possible side effects are symptomatic hypotension (dizziness), hyperkalemia, worsening renal function and cough. | |||
Possible side effects | |||
In patients with congestive HF, total mortality and hospitalization are significantly reduced by ACE inhibitors.<cite>18</cite> | |||
===Beta Blockers=== | |||
Beta blockade (in addition to an ACE inhibitor or ARB when ACE inhibitor is not tolerated) is indicated for every patient with symptomatic systolic HF and an EF ≤40 % (NYHA class II-IV) and in asymptomatic patients with a LVEF ≤40% after a MI. (Class I recommendation, level of evidence A).<cite>19</cite> | |||
* | Contraindications are: | ||
* | *Bronchial asthma | ||
*Second - or third degree heart block, sick sinus syndrome, sinus bradycardia | |||
==== | Beta blockers mainly exert their effect by reducing the toxic effects of the sympathetic nervous stimulation on the heart and by deactivating the renin-angiotensin system. | ||
Possible side effects include (symptomatic) hypotension, worsening of HF and bradycardia. The recommendation is ‘start low, go slow’, i.e. start with a low dose, and titrate every two weeks. | |||
In patients with persistent symptoms after treatment with a combination of beta blocker and ACE inhibitor or ARB, a mineralocorticoid/aldosterone receptor antagonist (MRA) is recommended. (Class I recommendation, level of evidence A) | |||
===Diuretics (Loop of Henle diuretics, Thiazides, Aldosterone antagonists)=== | |||
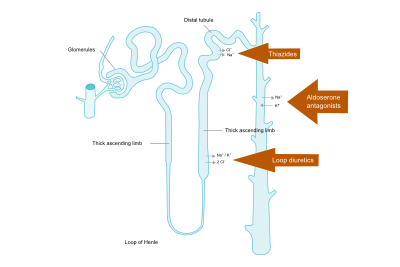
Possible side effects include symptomatic hypotension (dizziness), | [[Image:Henle_loop.svg|thumb|400px|'''Figure 6.''' Diuretics and site of action in the nephron.]] | ||
Diuretics reduce preload by venous vasodilatation and by increasing diuresis. As a result, filling pressures of the heart and the lung vasculature decrease. Although the effects of diuretics on mortality and morbidity have not been studied in patients with HF (irrespective of EF), it is recommended in patients with signs and symptoms of congestion as diuretics relieve dyspnea and edema. Figure 6 depicts the nephron and the sites where different diuretics work. | |||
====Loop of Henle diuretics==== | |||
Loop of Henle diuretics act on the ascending loop of Henle in the kidney tubules to inhibit sodium and chloride (and indirectly calcium and magnesium) reabsorption. This will ultimately result in increased urine production of sodium and water. Compared to thiazides, loop diuretics produce a more intense and shorter diuresis. | |||
====Thiazides==== | |||
Thiazide increases urine production by decreasing reabsorption of sodium in the distal tubule. This type of diuretic is often used in combination with loop diuretics to enhance their effects, but may be less effective in patients with a severely reduced kidney function. | |||
====Aldosterone antagonists==== | |||
Adding this drug is suggested for patients with moderate to severe symptomatic HF (NYHA class II to IV, refer to Table 2) and an LVEF < 35%. (Class I recommendation, level of evidence A) Contraindications: | |||
*Serum potassium concentration > 5.0 mmol/L | |||
*Serum creatinine > 220 µmol/L | |||
*Concomitant potassium sparing diuretic or potassium supplements | |||
*Combination of an ACEI and ARB | |||
Aldosterone antagonists reduce sodium retention by the kidney, and inhibit fibrosis formation in the heart. | |||
Possible side effects include hyperkalemia, hyponatremia, worsening renal function, and breast tenderness and/or enlargement. Eplerenon has less mastopathy side effects and is an alternative to spironolacton. In patients with severe heart failure, spironolactone in addition to standard therapy, reduces morbidity and mortality. <cite>20</cite> | |||
====Choice and combination of diuretics==== | |||
Patients with heart failure may be treated with a thiazide diuretic, which should be switched to a loop diuretic if a suboptimal response occurs. In patients with a decreased renal function, a loop diuretic is the mainstay of treatment. Addition of a thiazide diuretic to a loop diuretic can be considered in case of a suboptimal response of loop diuretic alone, when given in sufficient doses (furosemide 250 mg twice daily), suggesting that diuretic resistance is due to distal tubular increased activity of retaining sodium. In all patients with NYHA II or more, except in those with a creatinine clearance < 20 ml/min (creatinine > 220 micromol/L), addition of an aldosterone antagonist should be considered. In special cases in which hypercapnia plays a role, metabolic alkalosis can result from diuretics, and acetazolamide, a reversible carbonic anhydrase inhibitor, is then prescribed as an alternative diuretic. | |||
===Angiotensin receptor blockers (ARBs)=== | |||
ARBs are recommended in patients who do not tolerate an ACE inhibitor. Until recently, the addition of ARBs was the first choice recommendation in patients with HF and EF ≤40% who remained symptomatic despite optimal treatment with ACE inhibitor and beta blocker. As aldosterone antagonists have also proven their effects in NYHA class II patients, aldosterone antagonists have become first choice additional therapy after an ACE inhibitor and beta blocker. Whether ARBs may still be recommended in this patient group as added therapy after the addition of aldosterone antagonist is not known. | |||
Contraindications are: | |||
*Bilateral renal artery stenosis | |||
*Serum potassium concentration > 5.0 mmol/L | |||
*Serum creatine > 220 µmol/L | |||
*Severe aortic stenosis | |||
Possible side effects include symptomatic hypotension (dizziness), hyperkalemia, and a worsening renal function. | |||
===Digoxin=== | |||
In the past, digoxin was the standard treatment in HF. Digoxin inhibits sodium-potassium ATPase in the cell membrane of the myocytes, and, by decreasing the sodium extrusion, also inhibits the exchange of calcium out of the cell for sodium into the cell. More calcium remains in the myocyte and increases the contractility of the heart. In contrast to other inotropics, digoxin does not increase mortality. | |||
In patients with symptomatic HF and atrial fibrillation (AF) with a ventricular rate at rest of >80 beats per minute, use of digoxin may be considered to slow the ventricular rate. (Class I recommendation, level of evidence C) | |||
Digoxin may be considered to reduce HF hospitalization in patients with symptomatic (NYHA class II-IV) systolic HF in sinus rhythm with an EF ≤45%. These patients should also use an ACE inhibitor (or ARB) and an MRA (or ARB) and preferably a beta blocker. (Class IIb recommendation, level of evidence B) This may also be considered in patients with an EF ≤45% and persisting symptoms despite treatment with an ACE inhibitor (or ARB) and an MRA (or ARB). (Class IIb recommendation, level of evidence B) | |||
Contraindications for the use of digoxin are: | Contraindications for the use of digoxin are: | ||
* Second- or third degree heart block without a permanent pacemaker, sick sinus syndrome | *Second- or third degree heart block without a permanent pacemaker, sick sinus syndrome | ||
* Pre- | *Pre-excitation syndromes | ||
Possible side effects include sinoatrial or atrioventricular block, arrhythmias or signs of toxicity. | |||
Possible side effects include sinoatrial or atrioventricular block, arrhythmias or signs of toxicity (nausea and visual effects as halos). | |||
===Ivabradine=== | |||
Ivabradine lowers the heart rate through inhibition of the If channel in the sinus node. This drug can be used in patients who are still in NYHA class II-IV after treatment with ACE inhibitor (or ARB), beta blocker and an MRA (or ARB), who have a LVEF ≤35%, are in sinus rhythm and have a heart rate ≥75 beats/min. Ivabradine may also be used in patients who do not tolerate, or have contraindications for the use of beta blockers. | |||
===Hydralazine and isosorbide dinitrate (H-ISDN)=== | |||
H-ISDN can be used as an alternative treatment when both ACEI and ARBs are not tolerated by symptomatic HF patients with a LVEF ≤45% and dilated LV (or EF ≤35%). These patients should also receive a beta blocker and MRA. (Class IIb recommendation, level of evidence B). H-ISDN can be used in addition to the standard HF treatments (ACEI, beta blocker and MRA) in patients of Afro-American descent (Class IIb recommendation). H-ISDN may reduce risk of HF hospitalization and risk of premature death in patients with a LVEF ≤45% and dilated LV (or EF ≤35%) with persistent symptoms despite treatment with beta blocker, ACEI (or ARB), and an MRA (or ARB). (Class IIb recommendation, level of evidence B) | |||
Contraindications for the use of H-ISDN are: | |||
*Symptomatic hypotension | |||
*Lupus syndrome | |||
*Severe renal failure | |||
The H-ISDN combination acts by decreasing peripheral vascular resistance. | |||
Possible side effects include symptomatic hypotension or drug-induced lupus-like syndrome. | Possible side effects include symptomatic hypotension or drug-induced lupus-like syndrome. | ||
=== | ===Other=== | ||
*Anticoagulants | |||
*Anti platelet agents | |||
*Statins | |||
*Anti arrhythmic medication | |||
*Calcium antagonists | |||
==Therapy of acute heart failure== | |||
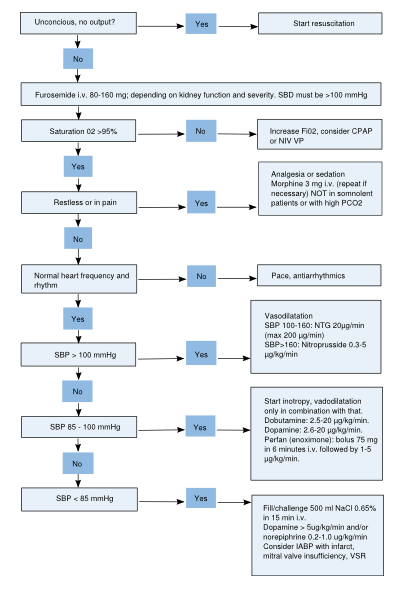
[[Image:acute_hf_flowchart.svg|400px|thumb|'''Figure 7.''' Flowchart acute HF.]] | |||
When severe symptoms of heart failure quickly develop over time, it is termed acute heart failure. In Table 6, common acute HF medications and their recommended doses are summarized. In Figure 7, a flowchart for the treatment of acute HF is depicted. The mainstay of acute heart failure therapy includes diuretics, vasodilators, inotropics and vasopressors. Moreover, oxygen and morphine can be added. | |||
{| class="wikitable" border="0" cellpadding="0" cellspacing="0" width="630px" | |||
|- | |||
!colspan="3"|Table 6. Medication in acute heart failure | |||
|- | |||
!Medication | |||
!Condition | |||
!Dose | |||
|- | |||
|'''Diuretics''' | |||
|colspan="2"|Adequate blood pressure and signs of overfilling | |||
|- | |||
| | |||
*Furosemide i.v. | |||
|valign="bottom"| | |||
Renal failure | |||
|40 mg | |||
125 mg – max 1000 mg | |||
|- | |||
| | |||
*Bumetanide i.v. | |||
|valign="bottom"| | |||
Renal failure | |||
|1 mg | |||
3 mg – max 25 mg | |||
|- | |||
|'''Vasodilators''' | |||
|colspan="2"|Adequate blood pressure and signs of severe overfilling | |||
|- | |||
| | |||
*Nitroglycerine i.v. | |||
| | |||
|20 µg/min – max 200 µg/min (guided by blood pressure) | |||
|- | |||
| | |||
*Nitroprusside i.v. | |||
||Hypertensive crisis or in combination with inotropic in case of a cardiogenic shock | |||
|0.3 µg/kg/min – max 5 µg/kg/min (guided by blood pressure) | |||
|- | |||
|colspan="3"|'''Inotropes''' | |||
|- | |||
| | |||
*Dobutamine i.v. | |||
|Low blood pressure and/or renal failure with or without overfilling | |||
|2-3 µg/kg/min – max 20 µg/kg/min | |||
|- | |||
| | |||
*Dopamine i.v. | |||
|Low blood pressure and/or renal failure with or without overfilling | |||
|2-3 µg/kg/min – max 20 µg/kg/min | |||
|- | |||
| | |||
*Enoximone i.v. | |||
|Signs of peripheral hypoperfusion with or without overfilling, and adequate blood pressure | |||
|0.25 – 0.75 mg/kg in 10 minutes; subsequently 1.25 – 7.5 µg/kg/min | |||
|- | |||
| | |||
*Levosimendan i.v. | |||
|If beta-blockade is thought to be contributing to hypoperfusion | |||
|0.1 µg/kg/min, | |||
can be decreased to 0.05 or increased to 0.2 µg/kg/min | |||
|- | |||
|colspan="3"|'''Vasopressors''' | |||
|- | |||
| | |||
*Adrenalin i.v. | |||
|Restore circulation in cardiogenic shock | |||
| | |||
|- | |||
| | |||
*Noradrenalin i.v. | |||
|Septic shock | |||
| | |||
|} | |||
Patient presents at first aid or emergency room with signs of acute HF. | |||
{| class="wikitable" border="0" cellpadding="0" cellspacing="0" width="630px" | |||
|- | |||
!colspan="3"|Table 7. Medication in chronic heart failure | |||
|- | |||
!Medication | |||
!Condition | |||
!Dose | |||
|- | |||
|'''Loop diuretic''' | |||
|Adequate blood pressure and signs of overfilling | |||
| | |||
|- | |||
|valign="top"| | |||
*Furosemide | |||
|valign="bottom"| | |||
Renal failure | |||
|40 mg | |||
80 mg – max 1000 mg | |||
|- | |||
|valign="top"| | |||
*Bumetanide | |||
|valign="bottom"| | |||
Renal failure | |||
|1 mg | |||
2 mg – max 25 mg | |||
|- | |||
|bgcolor="#F0F0F0" colspan="3"|'''ACE inhibitors''' | |||
|- | |||
|valign="top"| | |||
*Captopril | |||
| | |||
|Start 6.25mg | |||
1<sup>st</sup> week 6.25mg three times daily. | |||
3-5 weeks 12.5mg three times daily. | |||
>7 weeks 25mg three times daily. | |||
|- | |||
|valign="top"| | |||
*Lisinopril | |||
| | |||
|Start 2.5-5mg | |||
1<sup>st</sup> week 2.5-5mg twice daily. | |||
3-5 weeks 5-10mg twice daily. | |||
>7 weeks 10-20mg twice daily. | |||
|- | |||
|bgcolor="#F0F0F0 " colspan="3"|'''Beta blockers''' | |||
|- | |||
|valign="top"| | |||
*Metoprolol zoc (succinate) | |||
|valign="top"|EF >30-45% and NYHA II-III | |||
|Start 25mg | |||
1<sup>st</sup> week 50mg once daily. | |||
3-5 weeks 100mg once daily. | |||
>7 weeks 100-200mg once daily. | |||
|- | |||
| | |||
|valign="top"|EF <30% and NYHA IV | |||
|Start 12.5mg | |||
1<sup>st</sup> week 25mg once daily. | |||
3-5 weeks 50mg once daily. | |||
>7 weeks 100-200mg once daily. | |||
|- | |||
|valign="top"| | |||
*Bisoprolol | |||
|valign="top"|EF >30-45% and NYHA II-III | |||
|Start 2.5mg | |||
1<sup>st</sup> week 3.75mg once daily. | |||
3-5 weeks 5mg once daily. | |||
>7 weeks 7.5-10mg once daily. | |||
|- | |||
| | |||
|valign="top"|EF <30% and NYHA IV | |||
|Start 1.25mg | |||
1<sup>st</sup> week 2.5mg once daily. | |||
3-5 weeks 3.75mg once daily. | |||
>7 weeks 5-7.5-10mg once daily. | |||
|- | |||
|valign="top"| | |||
*Carvedilol | |||
|valign="top"|EF >30-45% and NYHA II-III | |||
|Start 6.25mg | |||
1<sup>st</sup> week 6.25mg twice daily. | |||
3-5 weeks 12.5mg twice daily. | |||
>7 weeks 25mg twice daily. | |||
|- | |||
| | |||
|valign="top"|EF <30% and NYHA IV | |||
|Start 3.125mg | |||
1<sup>st</sup> week 3.125mg twice daily. | |||
3-5 weeks 6.25mg twice daily. | |||
>7 weeks 12.5-25mg twice daily. | |||
|- | |||
|valign="top"| | |||
*Nebivolol | |||
|valign="top"|EF >30-45% and NYHA II-III | |||
|Start 1.25mg | |||
1<sup>st</sup> week 2.5mg once daily. | |||
3-5 weeks 5mg once daily. | |||
>7 weeks 10mg once daily. | |||
|- | |||
| | |||
|valign="top"|EF <30% and NYHA IV | |||
|Start 1.25mg | |||
1<sup>st</sup> week 2.5mg once daily. | |||
3-5 weeks 5mg once daily. | |||
>7 weeks 10mg once daily. | |||
|- | |||
|bgcolor="#F0F0F0 " colspan="3"|'''Aldosterone antagonist''' | |||
|- | |||
|valign="top"| | |||
*Spironolactone/eplerenone | |||
| | |||
|Start 25mg s.i.d. | |||
1<sup>st</sup> week potassium <5.0: 25mg once daily. | |||
potassium 5.0-5.5: 12.5mg once daily. | |||
potassium >5.5: stop | |||
3rd week potassium <5.0: 25mg once daily. | |||
potassium 5.0-5.5: 12.5mg once daily. | |||
potassium >5.5: stop | |||
|- | |||
|valign="top"| | |||
*Digoxin | |||
| | |||
|Start 0.5mg, 0.25mg and 0,25 mg, each with 6 hours in between | |||
Continue with 0.25mg once daily. | |||
Half dose with age above 70 or creatinin above 110 or with amiodarone use | |||
|- | |||
|bgcolor="#F0F0F0 " colspan="3"|'''AT II blockers''' | |||
|- | |||
|valign="top"| | |||
*Candesartan | |||
| | |||
|Start 4mg | |||
3-5 weeks 8mg once daily. | |||
>7 weeks 16mg once daily. | |||
|- | |||
|valign="top"| | |||
*Valsartan | |||
| | |||
|Start 40mg twice daily | |||
3-5 weeks 80mg twice daily. | |||
>7 weeks 160mg twice daily. | |||
|- | |||
|colspan="3" bgcolor="#F0F0F0 "|'''Hydralazine and isosorbide dinitrate (H-ISDN)''' | |||
|- | |||
|valign="top"| | |||
*Hydralazine | |||
| | |||
|Start 25mg three times daily. | |||
3-5 weeks 50mg three times daily. | |||
>7 weeks 75-100mg three times daily. | |||
|- | |||
|valign="top"| | |||
*ISDN | |||
| | |||
|Start 20mg twice daily | |||
3-5 weeks 40mg twice daily | |||
>7 weeks 80mg twice daily | |||
|} | |||
==Management of HF beyond medication== | |||
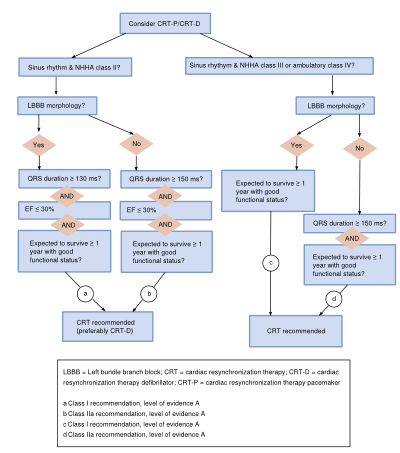
[[Image:CRT_flowchart.svg|thumb|400px|'''Figure 8.''' flowchart CRT]] | |||
===Device treatment=== | |||
Prevention of sudden death is an important goal in HF because approximately half of the deaths occur suddenly, and many of these are related to ventricular arrhythmias. Implantable cardioverter-defibrillator (ICD) therapy is recommended in survivors of cardiac arrest , irrespective of EF, when life expectancy is >1 year. (Class I recommendation, level of evidence A). | |||
In symptomatic HF patients (NYHA class II-III) with an EF ≤35% after more than 3 months of pharmacological treatment and a life expectancy >1 year, prophylactic ICD implantation is recommended in patients with ischemic etiology (Class I recommendation, level of evidence A) and non-ischemic etiology (Class I recommendation, level of evidence B). | |||
Cardiac resynchronization therapy (CRT) is indicated in patients with symptomatic heart failure with one type and severity of ventricular conduction delay (LBBB, QRS ≥120 msec), and preferably in patients with sinus rhythm. The responder rate (improvement of at least 5% EF) is about 70%. Recommendation for use of this therapy differs according to heart rhythm, NYHA class, QRS duration and morphology, and LVEF. This is depicted in the Figure 8. | |||
===Timing of ICD implantation=== | |||
Figure 5 offers recommendations to which patients should receive ICD treatment. In this flowchart, the timing of the placement has not been defined completely. In most patients, it should be safe to wait for their ICD whilst receiving (pharmacological) treatment as events typically occur after 6-12 months.<cite>12</cite> An exception to this rule is the group of high risk patients (i.e. patients with major myocardial infarction (MI), who have extensive fibrosis on the MRI or NSVT despite optimal pharmacological treatment); an ICD implantation should not be postponed too long in these patients. Early (within 40 days after event) ICD placement after an acute myocardial infarction has not been shown to reduce mortality, because the patients most at risk of sudden death are also the patients most at risk of death due to heart failure.<cite>13</cite><cite>14</cite><cite>15</cite> For this reason, prophylactic ICD treatment is recommended only after 40 days in post-infarct patients who have an EF < 35%. For non-ischemic heart failure patients, three months is considered a safe waiting time for an ICD. There are, however, also higher risk patients among this group, and a decision should be made for each patient on an individual basis.<cite>16</cite> | |||
===Heart transplantation and Left Ventricular Assist Devices=== | |||
When a patient has severe and progressive HF, his or her prognosis is grim. Considering the paucity of donor hearts, the waiting list for heart transplantation may be long and early consideration of heart transplantation is part of the treatment strategy in HF. Average 2-year survival rates after cardiac transplantation are approximately 80%. A patient in NYHA class III should be evaluated with an exercise test for maximal oxygen uptake, in order to consider further steps. Indication for heart transplantation includes a VO<sub>2max</sub> < 14 ml/min/kg.<cite>17</cite> | |||
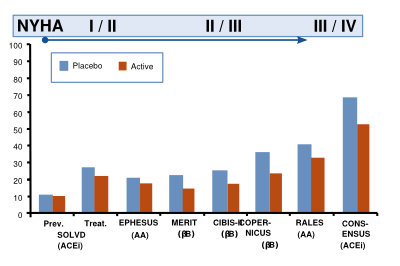
[[Image:HF_prognosis_trials.svg|thumb|right|400px|'''Figure 9.''' Two-year mortality in landmark contemporary clinical heart failure trials (from Cleland et al)]] | |||
Exclusion criteria are pulmonary hypertension (risk of immediate RV donor failure), severe comorbidity, and diabetes mellitus with organ damage. Left Ventricular Assist Devices are more commonly used as a bridge to transplantation, when the patient in on a waiting list. They have evolved from pulsatile to continuous flow pumps, with less complications and a longer durability. Often Left Ventricular Assist Devices become destination therapy. | |||
===Management of HF patients with preserved LVEF (HFPEF)=== | |||
To date, no evidence exists of any treatment reducing morbidity or mortality in this patient group. With the aim of controlling water and sodium retention and to decrease breathlessness and edema, diuretics are prescribed to HFPEF patients. Furthermore, ACE-I, Angiotensin II blockers and/or beta blockers may be considered. The CHARM trial including 3023 HF patients with preserved EF, showed angiotensin II blockade (candesartan) to have a moderate effect on hospital admission but showed no effect on the risk of cardiovascular death.<cite>8</cite> | |||
===Prognosis=== | |||
The life expectancy of a patient with heart failure is determined by age, NYHA class, LVEF, normal level of sodium, systolic blood pressure, use of medication and use of ICD or CRT-D ([http://depts.washington.edu/shfm/app.php Seattle Heart failure score]). The mean yearly annual mortality is approximately 10%, varying from <6% per year when a normal LVEF is identified, to > 14% per year with an EF of <15%. | |||
Trials with medication illustrate that the (short term) benefit of medication is highest when the NYHA class is higher (Figure 9).<cite>11</cite> | |||
==References== | |||
<biblio> | |||
#1 Withering W., Keys TE, An account of the foxglove and some of its medical uses, with practical remarks on dropsy, and other diseases, Classics of Cardiology. Volume I. New York, NY: Henry Schuman, Dover Publications; 1941: 231–252. | |||
#2 Kannel WB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for Estimating Risk of Heart Failure, Arch Intern Med. 1999;159:1197-204 | |||
#3 McMurray JJV et al., ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012, European Heart Journal (2012; 33;1787–1847) | |||
#4 McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJ, Dargie HJ. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997 Sep 20;350(9081):829-33 | |||
#5 Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003 Aug 26;108(8):977-82. Epub 2003 Aug 11. | |||
#6 Holubarsch C et al, Existence of the Frank-Starling Mechanism in the Failing Human Heart Investigations on the Organ, Tissue, and Sarcomere Levels. Circulation. 1996 Aug 15;94(4):683-9. | |||
#7 Mosterd A. Clinical epidemiology in heart failure. Heart 2007;93:1137-1146 | |||
#8 Yusuf S. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. The Lancet. Sept 2003 362;9386:777-781 | |||
#9 McMurray JJ. Clinical practice. Systolic heart failure. New England Journal of Medicine. 2010;362: 228-238 | |||
#10 McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. New England Journal of Medicine. 1971; 23;285(26):1441-6. | |||
#11 Cleland JGF, Andrew L, Clark AL. Delivering the cumulative benefits of triple therapy to improve outcomes in heart failure. Journal of the American College of Cardiology. 2003;42(7) | |||
#12 Moss AJ, Zareba W, Hall J, Klein H, Wilbur DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. New England Journal of Medicine. 2002;346:877–883. | |||
#13 Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. New England Journal of Medicine.2004;351:2481–2488 | |||
#14 Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, WojciechowskiD, Kornacewich-Jach Z, Sredniawa B, Lupkovics G, Hofgartner F, Lubinski A, Rosenqvist M, Habets A, Wegscheider K, Senges J. Defibrillator implantation early after myocardial infarction. New England Journal of Medicine. 2009;361:1427–1436 | |||
#15 Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, Burke M, MossAJ . Time-dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–1084. | |||
#16 Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with non-ischemic dilated cardiomyopathy. England Journal of Medicine. 2004;350:2151–2158 | |||
#17 Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786 | |||
#18 Garg R, Yusuf S, Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Journal of the American Medical Association. 1995;273:1450-6 | |||
#19 Foody JM, Farrell MH, Krumholz MH. ß-Blocker Therapy in Heart Failure. Scientific Review. Journal of the American Medical Association. 2002;287(7):883-889. | |||
#20 Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. New England Journal of Medicine .1999;341:709-17 | |||
</biblio> | |||
Latest revision as of 16:00, 11 October 2015
Ineke Nederend MSc,
Peter Damman MD,
W.E.M. Kok MD PhD
Introduction
History
In 1628, William Harvey first described the circulation. Before this time, there was very little understanding of the nature of heart failure (HF). There are, however, accounts of a disease that now would be called heart failure, and herbal medicines such as the ancient boiled bulb of squill, or, later on, the broom plant (Cytisus scoparius) and the foxglove (Digitalis purpura) were used as diuretics to treat heart failure or dropsy (edema). Foxglove was described as a diuretic by William Withering in 1785.[1] The essential glycoside substance digitalis of the leaves of the plant improves contractility of the cardiac muscle and has important parasympathetic effects, particularly on the atrioventricular node. In the 1950s, thiazide diuretics were introduced, and in the 1960s furosemide became available. For a long time, diuretics and digitalis were the main treatment options for HF. Vasodilator therapy for HF was introduced around 1960, and the first randomized trial showing a mortality benefit with nitrates and alphablockers for HF was published in 1986. In 1975, the first ACE inhibitor, captopril, was developed and it was approved for human use in 1981, with data from the first randomized trial being published in 1987. Spironolacton, introduced in 1959, was used (in low dose) for HF only after the introduction of ACE inhibitors. Beta blockers were hardly used in heart failure even though they were shown to beneficial in the 1970s. It was only in 1994 that data from the first randomized trial demonstrated a mortality benefit with beta blocker therapy.
Framingham heart study
In 1948, the Framingham heart study was launched. At its start, 5209 residents of the town Framingham in the USA, aged between 30 and 62 years, were included in the study in an attempt to determine risk factors for cardiac disease. The study is still in progress today and long term data from the lengthy follow up have been published. This study is considered to be the most important longitudinal source of data on the epidemiology of heart failure[2].
Definition and diagnosis
Definition of heart failure
The term heart failure (HF) (congestive heart failure or cardiac decompensation or decompensatio cordis) describes an acute or chronic situation in which the amount of blood pumped through the circulation by the heart, is insufficient to meet the body’s demands at a normal cardiac filling pressure. According to the guidelines of the European Society of Cardiology, HF is defined as a syndrome in which the patient has the following triad of features: (1) symptoms typical of HF; (2) signs typical of HF; and most importantly (3) objective evidence of a structural or functional abnormality of the heart at rest (Table 1).
| Table 1. Definition of heart failure |
|---|
| Heart failure is a clinical syndrome in which patients have the following features: |
|
And
|
And
|
Prevalence
The prevalence of HF in the Western world is estimated to be 1-2%, and the incidence is approximately 5-10 per 1000 persons per year (7,9). Coronary heart disease at a young age is more prevalent in men than women, and so the prevalence of HF is also higher in this group compared to age matched women. In older age groups, the prevalence of HF is equal between the sexes.
Acute and chronic heart failure
Heart failure may become a chronic condition, in which HF is persistent either with recurrences or with slow progression. A patient may be described as decompensated when chronic stable HF deteriorates. Acute HF has traditionally been used to describe the nature of the clinical presentation, as severe or of recent onset. Different clinical presentations fall under this definition.
Systolic versus diastolic heart failure
Heart failure patients may be broadly classified into one of two groups, or a combination of both, depending on the left ventricular ejection fraction (LVEF). The LVEF is most often assessed with echocardiography (see Table 3). When the LVEF is less than 45%, systolic pump function is abnormal and it is named systolic HF.[3] If LVEF is preserved (>50%), symptoms are attributed to impaired relaxation of the heart during diastole and therefore this condition is diagnosed as diastolic HF or HF with a preserved LVEF.[3][4] As a result of impaired relaxation, end diastolic pressure and, subsequently, left atrial- and pulmonary pressure will rise; alveolar pulmonary edema develops as a consequence of these changes. LF diastolic dysfunction may be present in asymptomatic patients, and it is considered an important precursor of heart failure.[5] Frequently, patients have both systolic and diastolic heart failure at the same time, but the term for this ailment is still systolic heart failure. The term heart failure is not limited to a failing left ventricle; the right ventricle may also be involved in the process and there may also be isolated right ventricular heart failure.
Pathophysiology of heart failure
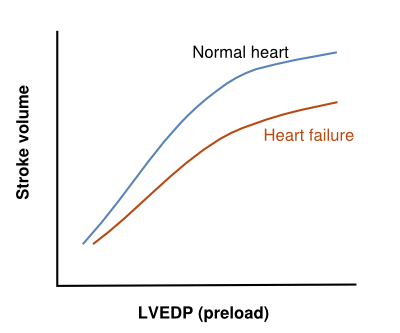
HF is caused by a loss of cardiac pump function, which can be due to a structural abnormality of the heart muscle (e.g. myocardial infarction) or a change in the heart function (and often structure) in response to an abnormal load (e.g. aortic valve stenosis). The relationship between loading the ventricle (by filling it) and its output was described by Frank and Starling in 1918 and has become the cornerstone in understanding heart failure and how to treat it. The relationship states that as a result of loading the heart (increasing its filling or its pressure), the output increases (Figure 1). A heart that has a lower output can be improved by increasing its volume and its loading pressure. This is what naturally happens (LV dilatation and increased filling pressure) when the heart does not pump out enough volume, and, in the first phase of disease, compensates for the loss of contractility. It takes more energy from the heart to work at an increased loading, but the heart has a reasonable energy reserve. In a chronic situation, remodeling of the heart progresses (by hypertrophy of the myocytes and dilatation by increasing myocyte length and matrix changes), which, in the long term, leads to a further loss in function. The result of this dysfunction is further increased loading pressures in the heart and, by communicating the diastolic loading pressures to the left atrium and pulmonary veins, the pulmonary capillaries may become overloaded and leak water into the lungs. This is the practical restriction of further filling the heart as a tool to improve its function; even poor left ventricles may be filled more to increase their output [6] but the patients’ pulmonary capillaries cannot tolerate these hydrostatic pressures and start to leak water.
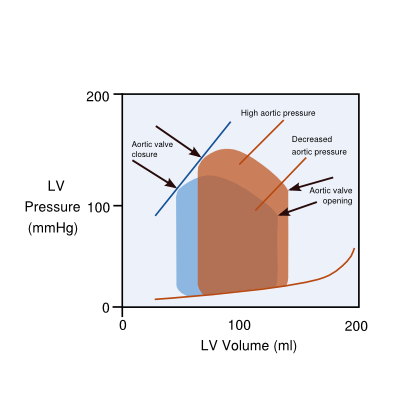
Hemodynamic explanations (the heart as a pump) use the concept of preload (filling) and afterload (workload of the heart, which is wall tension and arterial pressure or vascular resistance). In this way, the progression of left sided heart failure towards right sided heart failure is explained as follows: prolonged left ventricular failure increases pressures in the left atrium (preload), which in time leads to a subsequent increased resistance in the pulmonary vascular system (which is the afterload of the right ventricle) and eventually may also lead to right ventricular failure. Another relevant issue is afterload of the left ventricle influencing the output of the heart: as the afterload of the aortic pressure also influences the timing of closure of the aortic valve, a high aortic pressure will close the aortic valve early and will, therefore, diminish the output. Decreasing (theoretically diastolic, but more practically systolic) aortic pressure will increase the stroke volume by latter closure of the aortic valves. (Figure 2)
Hormonal/ sympathetic system mechanisms (RAAS/ Sympathetic overstimulation) of heart failure are as important as the hemodynamic mechanisms of heart failure.
A decreased cardiac output leads to diminished renal perfusion and release of hormones in the RAA-system: renin is released into the circulation by the renal juxtaglomerular apparatus, which stimulates the cleavage of angiotensinogen into angiotensin I and II during its passage through the lungs. Angiotensin II stimulates vasoconstriction in the kidneys, and in other vascular systems, increasing blood pressure; the second effect of angiotensin is to stimulate the release of aldosterone from the adrenals into the plasma, which retains sodium from the kidney tubules in the blood and thereby water. The RAA –system, which works as a compensatory mechanism for heart failure to increase blood pressure and blood volume, also stimulates hypertrophy of muscle cells and the formation of fibrosis, which in the long term are detrimental to heart failure.
The other compensatory mechanism for heart failure, stimulation of the sympathetic nervous system, increases heart rate to increase cardiac output, which is a powerful compensatory mechanism. However, chronic stimulation of the sympathetic nerves to the heart, leading to higher heart rates, is toxic to the heart, because of continuous release of norepinephrine to the myocyte. In addition, as a result of their continued stimulation, the betareceptors for norepinephrine are downregulated in heart failure, which further diminishes the function and functional reserve of the heart.
Management
When a patient presents with symptoms of heart failure, it is worthwhile to have a dedicated diagnostic and therapeutic plan, in the order as indicated below (Figure 3). Clinical aspects are important for diagnosis, but the final diagnosis is only made after objective evidence of heart dysfunction.
Clinical aspects
History
A careful history of the patient is important for the diagnosis and in order to identify the cause of HF. The history (and physical examination) can be used to differentiate between the abovementioned potential causes of HF (refer to Etiology of heart failure). Family history of HF, smoking status, hyperlipidaemia, hypertension and diabetes mellitus are factors that should be taken into account during the assessment of the patient history in order to draw a risk profile of the patient. Finally, the history should include previous events and the response to therapy.
Symptoms and signs
HF can manifest with a multitude of different symptoms and signs, but shortness of breath and tiredness are the most characteristic. The Framingham Heart Study defined major and minor diagnostic criteria for HF.
Major criteria:
- Paroxysmal nocturnal dyspnea
- Neck vein distention
- Pulmonaty rales
- Radiographic cardiomegaly (increasing heart size on chest radiography)
- Acute pulmonary edema
- S3 gallop
- Increased central venous pressure (>16 cm H2O at right atrium)
- Hepatojugular reflux
- Weight loss >4.5 kg in 5 days in response to treatment
Minor criteria:
- Bilateral ankle edema
- Nocturnal cough
- Dyspnea on ordinary exertion
- Hepatomegaly
- Pleural effusion
- Tachycardia (heart rate>120 beats/min.)
Minor criteria are acceptable only if they cannot be attributed to another medical condition (such as pulmonary hypertension, chronic lung disease, cirrhosis, ascites, or the nephrotic syndrome).
Diagnosis of HF requires the simultaneous presence of at least 2 major criteria or 1 major criterion in conjunction with 2 minor criteria. The Framingham Heart Study criteria are 100% sensitive and 78% specific for identifying persons with definite congestive heart failure in an outpatient population.[7]
Severity of HF
In general, correlation between the severity of symptoms and the severity of HF in terms of loss of maximal oxygen consumption is weak.[3] The New York Heart Association functional classification is used most frequently to classify the severity of HF (Table 2). Assessing severity is needed for the proper therapy/ medication to be chosen.
| Table 2. NYHA functional classification | |
|---|---|
| Severity based on symptoms and physical activity | |
| Class I | No limitation of physical activity.
Ordinary physical activity does not cause undue fatigue, palpitation, or dyspnoea. |
| Class II | Slight limitation of physical activity.
Comfortable at rest, but ordinary physical activity results in fatigue, palpitation, or dyspnoea. |
| Class III | Marked limitation of physical activity.
Comfortable at rest, but less than ordinary activity results in fatigue, palpitation, or dyspnoea. |
| Class IV | Unable to carry on any physical activity without discomfort.
Symptoms at rest. If any physical activity is undertaken, discomfort is increased. |
Physical examination
There are several key features in the clinical examination of a patient presenting with HF. The physical examination should focus on the general appearance of the patient, pulse and blood pressure, signs of fluid overload (increased jugular venous pressure, peripheral edema, ascites and hepatomegaly), the lungs, and the heart (apex, Gallop rhythm, third heart sound, murmurs).
Additional diagnostic tests
In order to assist in the diagnosis of HF and to differentiate between possible causes of HF, the following tests are available.
Electrocardiogram
An electrocardiogram (ECG) should be performed on every patient suspected of HF. Several common abnormalities (including possible causes) indicative of HF on the ECG include but are not limited to: sinus tachy- or bradycardia, atrial tachycardia, -flutter, or –fibrillation, ventricular arrhythmias, ischemia (including myocardial infarction), abnormal Q waves, left ventricular hypertrophy, micro voltages, and QRS length >120 ms. Although an abnormal ECG (excluding arrhythmias) has a low positive predictive value for HF, a normal ECG is highly indicative of the absence of HF.
Chest X-ray
A chest X-ray is a part of the standard examination in potential HF patients. Importantly, the X-ray is a tool to detect cardiomegaly (defined as a cardiac: thoracic ratio of > 0,5) or other clues (redistribution, Kerley B-lines and pleural effusion) that indicate HF. It is also important to rule out other causes of dyspnea.
Echocardiography
Echocardiography is the cornerstone in the diagnosis of HF, and should be performed routinely, because ventricular function can be evaluated accurately with this technique. It can provide objective evidence of a structural or functional abnormality of the heart at rest, besides signs and symptoms that are typical of heart failure. Important parameters that can be assessed include, but are not limited to, wall motion, valve function, left ventricular ejection fraction and diastolic function. Diastolic dysfunction might be an important finding in symptomatic patients with a preserved ejection fraction. Refer to Table 3 for common echocardiographic findings in HF. Transesophageal echocardiography is indicated in patients with an inadequate transthoracic echo window, suspected endocarditis, complicated valvular disease or to exclude a LV thrombus. If echocardiography provides inadequate information or in patients with suspected coronary artery disease, additional imaging includes CT scanning, cardiac magnetic resonance imaging or radionuclide imaging.
Laboratory tests
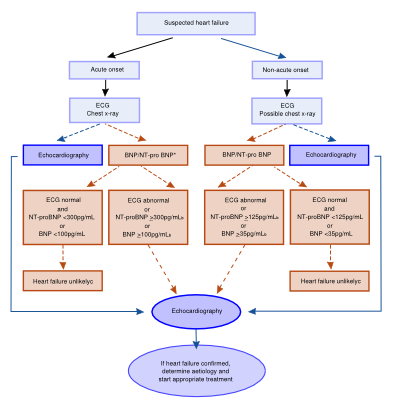
A standard blood assessment includes a complete blood count, electrolytes, renal function, glucose and liver function. Furthermore, urinalysis and other tests, depending on the clinical condition of the patient, complete the laboratory assessment. For example, cardiac troponins must be sampled if an ACS is in the differential diagnosis. In patients suspected of HF, values of natriuretic peptides (such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP)) can provide important information regarding the diagnosis, management and prognosis of HF. Natriuretic peptides are enzymes, secreted by the atria or ventricles in response to myocardial wall stress. The most commonly used tests are BNP and NT-proBNP measurements, which despite their different half-lives in the plasma, do not differ substantially in terms of diagnostic ability. Cut-off values are different in acute settings with acute dyspnea compared to chronic settings. Normal values are almost 100% specific, and exclude heart failure in patients >18 year old. Abnormal values do not have a 100% predictive value, and objective evidence for heart failure is still needed. The values for BNP and NTproBNP are also used to evaluate the prognosis in patients with known HF, in whom higher values carry a worse prognosis.
Exercise test
An exercise test is not diagnostic for HF, but may be used to identify ischemia as the cause of heart failure, or it can be used to assess the severity of HF, usually in conjunction with maximal oxygen uptake (VO2max) measurement. This test is performed on a treadmill or on a bicycle ergo meter. The patient is asked to give maximal effort while the workload gradually increases. During the test, the ECG is monitored for ischemia. When possible, oxygen consumption should also be measured during the test. Not only is an oxygen consumption test a good tool to discriminate between lung- peripheral- or heart problems, but the obtained value for maximal oxygen uptake (VO2max) has an important prognostic value.
Heart catheterization
Heart catheterization is not always part of the routine diagnosis and work-up of patients with HF. It should be considered however to exclude coronary heart disease (Class of recommendation IIa, level of evidence C, see Table 4). Coronary angiography is recommended in patients at high risk of coronary artery disease (Class of recommendation I, level of evidence C) and in HF patients with significant valvular disease (Class of recommendation IIa, level of evidence C).
| Table 3. Common echocardiographic abnormalities in heart failure | ||
|---|---|---|
| Measurement | Abnormality | Clinical implications |
| Left ventricular ejection fraction (LVEF) | Reduced (< 50%) | Left ventricular global systolic dysfunction |
| Left ventricular wall motion | Akinesis, hypokinesis, dyskinesis | Myocardial infarction/ischaemia,
Cardiomyopathy, Myocarditis |
| Left ventricular end-diastolic diameter | Increased (≥60 mm/>32 mm/m2)) | Volume overload HF likely |
| Left ventricular end-systolic diameter | Increased (≥45 mm/>25 mm/m2,) | Volume overload
HF likely |
| Left ventricular fractional shortening | Reduced (<25%) | Left ventricular systolic dysfunction |
| Left atrial volume index | Increased (volume >34 mL/m2) | Increased filling pressures,
Mitral valve dysfunction |
| Left ventricular thickness | Hypertrophy (>11 – 12 mm) | Hypertention,
Aortic stenosis, Hypertrophic cardiomyopathy |
| Valvular structure and function | Valvular stenosis or regurgitation (especially aortic stenosis and mitral insufficiency) | May be primary cause of HF or complicating factor
Asses haemodynamic consequences Consider surgery |
| Mitral diastolic flow profile | Abnormalities of the early and late diastolic filling patterns | Indicates diastolic dysfunction and suggests mechanism |
| Tricuspid regurgitation peak velocity | Increased (>3.4 m/s) | Increased right ventricular systolic pressure |
| Pericardium | Effusion,
Haemopericardium, Calcification |
Consider tamponade,
Malignancy, Systemic disease, Acute or chronic pericarditis, Constrictive pericarditis |
| Aortic outflow velocity time integral | Reduced (<15 cm) | Reduced low stroke volume |
| Right ventricular function (e.g. TAPSE) | Reduced (TAPSE < 16 mm) | RV systolic dysfunction |
| Inferior vena cava | Dilated, with no respiratory collapse | Increased right atrial pressures,
Right ventricular dysfunction, Volume overload Pulmonary hypertention possible |
Etiology of heart failure
Coronary heart disease
The most important cause (50% of the cases) of HF in the Western world is ischemic heart disease, including myocardial infarction. These patients mainly suffer from systolic HF due to wall motion abnormalities of the affected area and re-modeling of the non-affected parts of the myocardium.
Hypertension
In patients with a high systolic blood pressure (BP), the left ventricle faces an increased afterload (a higher workload pumping the blood against the increased vascular resistance). Over a certain period of time, this will lead to hypertrophy of the cardiac myocardium, and longer term remodeling may lead to pump function disorders (diastolic or systolic). In as many as 60-70% of patients suffering HF, hypertension is the primary or secondary cause of the condition.
Heart rhythm disorders
Atrial fibrillation is a common rhythm disorder in the elderly. With this condition, the atria do not contract in the coordinated fashion as they would in normal sinus rhythm, and therefore the atria never optimally empty. Normally, the ‘atrial kick’ contributes approximately 15% of the stroke volume. The absence of the atrial kick during atrial fibrillation can contribute to a reduced LVEF. However, atrial fibrillation is seldom the cause of heart failure, but more often a trigger of heart failure in already existing structural heart disease.
Valvular disease
Valvular disease, especially mitral- or aortic, can cause volume and pressure overload of the left ventricle of the heart. This overload causes dilation and / or hypertrophy of the left ventricle, which in the long term decreases the pump function.
Cardiomyopathies
Dilated cardiomyopathy (DCM) is characterized by dilatation of one or both of the ventricles of the heart, with a general decrease in contractility and consequently a decreased pump function. In approximately 30% of the cases, DCM is hereditary.
Hypertrophic cardiomyopathy (see also Hypertension) is characterized by hypertrophy, which may be concentric or asymmetric. The asymmetric form is usually hereditary.
Restrictive cardiomyopathy is characterized by a primary diastolic dysfunction of one or more of the ventricles, leading to increased filling pressures and hypertrophy, and initially a preserved systolic function.
Arrhythmogenic right ventricular cardiomyopathy is characterized by fatty infiltration and fibrosis of the right ventricle or the left ventricle or both and is usually hereditary.
Pericardial disease and Tamponade
Restriction of ventricular filling by a tight (inflamed or constrictive) pericardium or by pericardial effusion and tamponade can be the cause of diastolic HF.
Drugs
- Drugs that can cause HF are:
- Cytotoxic agents (chemotherapy, especially doxorubicin)
- The antipsychotic agent clozapine
- Drugs that can aggravate HF are:
- Beta blockers
- Calcium antagonist
- Antiarrhythmics
- Disulfiram
Toxins
- Alcohol
- Cocaine,
- Trace elements (mercury, cobalt, arsenic).
Endocrine disorders
- Diabetes mellitus
- Hypo- or hyperthyroidism
- Cushing syndrome
- Adrenal insufficiency
- Excessive growth hormone in acromegaly
- Phaeochromocytoma
Nutritional status
Deficiency of thiamine, selenium, or camitine, in states of severe cachexia.
Infiltrative and storage disorders
- Sarcoidosis
- Amyloidosis
- Haemochromatosis
- Connective tissue disease
Infectious disease
- Chagas’ disease
- HIV infection
- Viral, bacterial or protozoal diseases causing myocarditis.
Management in investigating etiology of heart failure
- Assess globally: are there triggers of heart failure. Hypertension, infection, anemia, rhythm disorders. Perform standard laboratory tests: in addition to hemoglobin, leukocytes, thrombocytes, creatinin, sodium and potassium levels, also liver function, thyroid function (TSH), glucose.
- Assess ischemia: are there indications of ischemic etiology (ECG: Q’s or significant and changing ST segments, laboratory: troponins, and echocardiogram: segmental wall motion abnormality in coronary territory areas) ? If yes, then proceed with further coronary artery or myocardial perfusion imaging.
- Are there no indications for ischemic etiology?
- Classify phenotype of cardiomyopathy: dilated, hypertrophic, restrictive, arrhytmogenic right ventricular cardiomyopathy.
- Then assess with additional laboratory tests, including creatinin kinase, autoimmune markers, eosinophilia, ferritin and iron saturation. In some suspected cases: calcium and albumin.
- Look for clues of etiologies: history, family history (including maternal inheritage of diabetes in a family)
- In case of fever look for infectious etiology, MRI confirmation for possible myocarditis, plasma serology.
- Look for clues on ECG: microvoltage on the ECG, AV block in combination with later atrial fibrillation. Additional lab may be warranted (monoclonal proteins in case of microvoltage in the presence of sufficient amounts of myocardium and the absence of pericardial fluid or pulmonary emphysema)
- MRI for further classification of cardiomyopathy and assessment of presence and localization of delayed contrast enhancement
- Coronary arteriography or coronary CT scan to exclude coronary artery disease
- Myocardial biopsy in cases where the suspicion of severe underlying disease is high (e.g. fulminant myocarditis, sarcoidosis suspicion on MRI with no other organ involved).
- Genetic testing after counseling
Therapy of heart failure
The therapeutic management of HF involves both pharmacological and non-pharmacological treatment. The goal is reduction in mortality and morbidity, prevention of the progression of HF, and the treatment of (non-) cardiovascular co-morbidities.
Non-pharmacological treatment
Non-pharmacological management is of great importance for HF patients. It can have a significant impact on symptoms, functional capacity, wellbeing, morbidity, and prognosis. The most important non-pharmacological options are described below.
Education
Education of both the patient and their family about HF and its symptoms is important. The patient and/or the caregiver should be able to undertake appropriate actions such as adjusting the diuretic dose or contact the physician when necessary. (Class I recommendation, level of evidence C; see Table 4) Education on the importance and (side) effects of medication should be provided to the patient in order to increase compliance. (Class I recommendation, level of evidence C)
Fluid and sodium restriction
In patients with severe symptoms of HF, restriction of fluid intake (to 1500 ml/day) may be considered. (Class IIa recommendation, level of evidence C). Also, patients should be educated about the salt content of food and advised to minimize their salt intake (< 2 gram/ day) in order to prevent fluid retention. (Class I recommendation, level of evidence C)
Body weight
CHF patients should carefully monitor their body weight. A sudden increase in weight is a potential consequence of fluid retention and deterioration of HF. If patients notice a weight gain of >2kg in 3 days, they should consult their physician. (Class I recommendation, level of evidence C). In obese patients (body mass index >30 kg/m2), weight reduction should be promoted to prevent progression of HF, decrease symptoms and improve the overall wellbeing of the patient. (Class IIa recommendation, level of evidence C). Also, attention should be paid to weight loss due to malnutrition, which is frequently observed in severe HF. An altered metabolism, inflammatory mechanisms or a decreased food intake may be important factors in the pathophysiology of cardiac cachexia in HF. (Class I recommendation, level of evidence C)
Alcohol and tobacco
Alcohol intake should be minimized, as it may increase blood pressure and/or have a negative inotropic effect. (Class IIa recommendation, level of evidence C). Smoking cessation should be encouraged. It is recommended that patients with HF receive support and advice on this topic. (Class I recommendation, level of evidence C). A reduction in alcohol and tobacco intake might also improve co-morbidities, including sleep disorders.
Exercise
Exercise training is recommended to all chronic stable HF patients. Twenty years ago, exercise was strongly discouraged in patients with HF as it was considered to be harmful. Nowadays, numerous studies have demonstrated the opposite. Rehabilitation programs have shown to increase exercise capacity and health related quality of life, and decrease hospitalization rates and symptoms. (Class I recommendation, level of evidence A)
Other
Other non-pharmacological treatment recommendations include immunization of HF patients (pneumococcal- and influenza vaccination should be considered), the consulting of a physician around pregnancy, the screening for depression and sleep disorders that require additional medical attention.
Pharmacological treatment
A flowchart for the treatment of patients presenting with systolic HF is depicted in Figure 5. Medications with a class I indication in patients with systolic heart failure are summarized in Table 5. Indications, mode of action, contraindications of the medication, and possible side effects of drugs included in this algorithm are discussed below.
| NYHA I & EF <40% | NYHA II | NYHA III | NYHA IV | |
| Diuretic | ||||
| ACE-inhib | ||||
| AT-II antagonist | Alternative | Alternative | Alternative | |
| Betablocker | Post infarction | |||
| Aldosteron antagonist | EF < 35% | EF < 35% | EF < 35% | |
| Nitrate / Hydralazine | Afro-American | Afro-American | Afro-American | |
| Ivabradine | SR>75/min & EF<35% | SR>75/min & EF<35% | SR>75/min & EF<35% | |
| Digoxin | ||||
| Table 5. Medication with a class I indication in patients with systolic heart failure. Note that AT-II antagonists are alternative medicine for ACE inhibitors in case of intolerance (coughing, allergy). Nitrates and Hydralazine are added therapy for patients of Afro-American descent, and alternative therapy for patients that cannot tolerate ACE-inhibitors and AT-II antagonists). Digoxin can also be seen as symptomatic (instead of added preventive) treatment, not always necessary in NYHA III or even IV. | ||||
Angiotensin-converting enzyme (ACE) inhibitors
An ACE inhibitor is indicated for every patient with symptomatic systolic HF and an EF ≤40 % (NYHA class II-IV). (Class I recommendation, level of evidence A) Contraindications for the use of ACE inhibitors are:
- History of angioedema
- Bilateral renal artery stenosis
- Serum potassium concentration >5.0 mmol/L
- Serum creatinine >220 µmol/L
- Severe aortic stenosis
ACE inhibitors relieve the heart by decreasing the preload and afterload. This is achieved through two mechanisms. Firstly, conversion of angiotensin-I to angiotensin– II is inhibited, which reduces vasoconstriction and lowers BP. Secondly, production of aldosterone is decreased, as angiotensin II induces this production. Aldosterone stimulates sodium- and water retention. Possible side effects are symptomatic hypotension (dizziness), hyperkalemia, worsening renal function and cough.
In patients with congestive HF, total mortality and hospitalization are significantly reduced by ACE inhibitors.[8]
Beta Blockers
Beta blockade (in addition to an ACE inhibitor or ARB when ACE inhibitor is not tolerated) is indicated for every patient with symptomatic systolic HF and an EF ≤40 % (NYHA class II-IV) and in asymptomatic patients with a LVEF ≤40% after a MI. (Class I recommendation, level of evidence A).[9] Contraindications are:
- Bronchial asthma
- Second - or third degree heart block, sick sinus syndrome, sinus bradycardia
Beta blockers mainly exert their effect by reducing the toxic effects of the sympathetic nervous stimulation on the heart and by deactivating the renin-angiotensin system.
Possible side effects include (symptomatic) hypotension, worsening of HF and bradycardia. The recommendation is ‘start low, go slow’, i.e. start with a low dose, and titrate every two weeks.
In patients with persistent symptoms after treatment with a combination of beta blocker and ACE inhibitor or ARB, a mineralocorticoid/aldosterone receptor antagonist (MRA) is recommended. (Class I recommendation, level of evidence A)
Diuretics (Loop of Henle diuretics, Thiazides, Aldosterone antagonists)
Diuretics reduce preload by venous vasodilatation and by increasing diuresis. As a result, filling pressures of the heart and the lung vasculature decrease. Although the effects of diuretics on mortality and morbidity have not been studied in patients with HF (irrespective of EF), it is recommended in patients with signs and symptoms of congestion as diuretics relieve dyspnea and edema. Figure 6 depicts the nephron and the sites where different diuretics work.
Loop of Henle diuretics
Loop of Henle diuretics act on the ascending loop of Henle in the kidney tubules to inhibit sodium and chloride (and indirectly calcium and magnesium) reabsorption. This will ultimately result in increased urine production of sodium and water. Compared to thiazides, loop diuretics produce a more intense and shorter diuresis.
Thiazides
Thiazide increases urine production by decreasing reabsorption of sodium in the distal tubule. This type of diuretic is often used in combination with loop diuretics to enhance their effects, but may be less effective in patients with a severely reduced kidney function.
Aldosterone antagonists
Adding this drug is suggested for patients with moderate to severe symptomatic HF (NYHA class II to IV, refer to Table 2) and an LVEF < 35%. (Class I recommendation, level of evidence A) Contraindications:
- Serum potassium concentration > 5.0 mmol/L
- Serum creatinine > 220 µmol/L
- Concomitant potassium sparing diuretic or potassium supplements
- Combination of an ACEI and ARB
Aldosterone antagonists reduce sodium retention by the kidney, and inhibit fibrosis formation in the heart.
Possible side effects include hyperkalemia, hyponatremia, worsening renal function, and breast tenderness and/or enlargement. Eplerenon has less mastopathy side effects and is an alternative to spironolacton. In patients with severe heart failure, spironolactone in addition to standard therapy, reduces morbidity and mortality. [10]
Choice and combination of diuretics
Patients with heart failure may be treated with a thiazide diuretic, which should be switched to a loop diuretic if a suboptimal response occurs. In patients with a decreased renal function, a loop diuretic is the mainstay of treatment. Addition of a thiazide diuretic to a loop diuretic can be considered in case of a suboptimal response of loop diuretic alone, when given in sufficient doses (furosemide 250 mg twice daily), suggesting that diuretic resistance is due to distal tubular increased activity of retaining sodium. In all patients with NYHA II or more, except in those with a creatinine clearance < 20 ml/min (creatinine > 220 micromol/L), addition of an aldosterone antagonist should be considered. In special cases in which hypercapnia plays a role, metabolic alkalosis can result from diuretics, and acetazolamide, a reversible carbonic anhydrase inhibitor, is then prescribed as an alternative diuretic.
Angiotensin receptor blockers (ARBs)
ARBs are recommended in patients who do not tolerate an ACE inhibitor. Until recently, the addition of ARBs was the first choice recommendation in patients with HF and EF ≤40% who remained symptomatic despite optimal treatment with ACE inhibitor and beta blocker. As aldosterone antagonists have also proven their effects in NYHA class II patients, aldosterone antagonists have become first choice additional therapy after an ACE inhibitor and beta blocker. Whether ARBs may still be recommended in this patient group as added therapy after the addition of aldosterone antagonist is not known.
Contraindications are:
- Bilateral renal artery stenosis
- Serum potassium concentration > 5.0 mmol/L
- Serum creatine > 220 µmol/L
- Severe aortic stenosis
Possible side effects include symptomatic hypotension (dizziness), hyperkalemia, and a worsening renal function.
Digoxin
In the past, digoxin was the standard treatment in HF. Digoxin inhibits sodium-potassium ATPase in the cell membrane of the myocytes, and, by decreasing the sodium extrusion, also inhibits the exchange of calcium out of the cell for sodium into the cell. More calcium remains in the myocyte and increases the contractility of the heart. In contrast to other inotropics, digoxin does not increase mortality.
In patients with symptomatic HF and atrial fibrillation (AF) with a ventricular rate at rest of >80 beats per minute, use of digoxin may be considered to slow the ventricular rate. (Class I recommendation, level of evidence C)
Digoxin may be considered to reduce HF hospitalization in patients with symptomatic (NYHA class II-IV) systolic HF in sinus rhythm with an EF ≤45%. These patients should also use an ACE inhibitor (or ARB) and an MRA (or ARB) and preferably a beta blocker. (Class IIb recommendation, level of evidence B) This may also be considered in patients with an EF ≤45% and persisting symptoms despite treatment with an ACE inhibitor (or ARB) and an MRA (or ARB). (Class IIb recommendation, level of evidence B)
Contraindications for the use of digoxin are:
- Second- or third degree heart block without a permanent pacemaker, sick sinus syndrome
- Pre-excitation syndromes
Possible side effects include sinoatrial or atrioventricular block, arrhythmias or signs of toxicity (nausea and visual effects as halos).
Ivabradine
Ivabradine lowers the heart rate through inhibition of the If channel in the sinus node. This drug can be used in patients who are still in NYHA class II-IV after treatment with ACE inhibitor (or ARB), beta blocker and an MRA (or ARB), who have a LVEF ≤35%, are in sinus rhythm and have a heart rate ≥75 beats/min. Ivabradine may also be used in patients who do not tolerate, or have contraindications for the use of beta blockers.
Hydralazine and isosorbide dinitrate (H-ISDN)
H-ISDN can be used as an alternative treatment when both ACEI and ARBs are not tolerated by symptomatic HF patients with a LVEF ≤45% and dilated LV (or EF ≤35%). These patients should also receive a beta blocker and MRA. (Class IIb recommendation, level of evidence B). H-ISDN can be used in addition to the standard HF treatments (ACEI, beta blocker and MRA) in patients of Afro-American descent (Class IIb recommendation). H-ISDN may reduce risk of HF hospitalization and risk of premature death in patients with a LVEF ≤45% and dilated LV (or EF ≤35%) with persistent symptoms despite treatment with beta blocker, ACEI (or ARB), and an MRA (or ARB). (Class IIb recommendation, level of evidence B)
Contraindications for the use of H-ISDN are:
- Symptomatic hypotension
- Lupus syndrome
- Severe renal failure
The H-ISDN combination acts by decreasing peripheral vascular resistance.
Possible side effects include symptomatic hypotension or drug-induced lupus-like syndrome.
Other
- Anticoagulants
- Anti platelet agents
- Statins
- Anti arrhythmic medication
- Calcium antagonists
Therapy of acute heart failure
When severe symptoms of heart failure quickly develop over time, it is termed acute heart failure. In Table 6, common acute HF medications and their recommended doses are summarized. In Figure 7, a flowchart for the treatment of acute HF is depicted. The mainstay of acute heart failure therapy includes diuretics, vasodilators, inotropics and vasopressors. Moreover, oxygen and morphine can be added.
| Table 6. Medication in acute heart failure | ||
|---|---|---|
| Medication | Condition | Dose |
| Diuretics | Adequate blood pressure and signs of overfilling | |
|
Renal failure |
40 mg
125 mg – max 1000 mg |
|
Renal failure |
1 mg
3 mg – max 25 mg |
| Vasodilators | Adequate blood pressure and signs of severe overfilling | |
|
20 µg/min – max 200 µg/min (guided by blood pressure) | |
|
Hypertensive crisis or in combination with inotropic in case of a cardiogenic shock | 0.3 µg/kg/min – max 5 µg/kg/min (guided by blood pressure) |
| Inotropes | ||
|
Low blood pressure and/or renal failure with or without overfilling | 2-3 µg/kg/min – max 20 µg/kg/min |
|
Low blood pressure and/or renal failure with or without overfilling | 2-3 µg/kg/min – max 20 µg/kg/min |
|
Signs of peripheral hypoperfusion with or without overfilling, and adequate blood pressure | 0.25 – 0.75 mg/kg in 10 minutes; subsequently 1.25 – 7.5 µg/kg/min |
|
If beta-blockade is thought to be contributing to hypoperfusion | 0.1 µg/kg/min,
can be decreased to 0.05 or increased to 0.2 µg/kg/min |
| Vasopressors | ||
|
Restore circulation in cardiogenic shock | |
|
Septic shock | |
Patient presents at first aid or emergency room with signs of acute HF.
| Table 7. Medication in chronic heart failure | ||
|---|---|---|
| Medication | Condition | Dose |
| Loop diuretic | Adequate blood pressure and signs of overfilling | |
|
Renal failure |
40 mg
80 mg – max 1000 mg |
|
Renal failure |
1 mg
2 mg – max 25 mg |
| ACE inhibitors | ||
|
Start 6.25mg
1st week 6.25mg three times daily. 3-5 weeks 12.5mg three times daily. >7 weeks 25mg three times daily. | |
|
Start 2.5-5mg
1st week 2.5-5mg twice daily. 3-5 weeks 5-10mg twice daily. >7 weeks 10-20mg twice daily. | |
| Beta blockers | ||
|
EF >30-45% and NYHA II-III | Start 25mg
1st week 50mg once daily. 3-5 weeks 100mg once daily. >7 weeks 100-200mg once daily. |
| EF <30% and NYHA IV | Start 12.5mg
1st week 25mg once daily. 3-5 weeks 50mg once daily. >7 weeks 100-200mg once daily. | |
|
EF >30-45% and NYHA II-III | Start 2.5mg
1st week 3.75mg once daily. 3-5 weeks 5mg once daily. >7 weeks 7.5-10mg once daily. |
| EF <30% and NYHA IV | Start 1.25mg
1st week 2.5mg once daily. 3-5 weeks 3.75mg once daily. >7 weeks 5-7.5-10mg once daily. | |
|
EF >30-45% and NYHA II-III | Start 6.25mg
1st week 6.25mg twice daily. 3-5 weeks 12.5mg twice daily. >7 weeks 25mg twice daily. |
| EF <30% and NYHA IV | Start 3.125mg
1st week 3.125mg twice daily. 3-5 weeks 6.25mg twice daily. >7 weeks 12.5-25mg twice daily. | |
|
EF >30-45% and NYHA II-III | Start 1.25mg
1st week 2.5mg once daily. 3-5 weeks 5mg once daily. >7 weeks 10mg once daily. |
| EF <30% and NYHA IV | Start 1.25mg
1st week 2.5mg once daily. 3-5 weeks 5mg once daily. >7 weeks 10mg once daily. | |
| Aldosterone antagonist | ||
|
Start 25mg s.i.d.
1st week potassium <5.0: 25mg once daily. potassium 5.0-5.5: 12.5mg once daily. potassium >5.5: stop 3rd week potassium <5.0: 25mg once daily. potassium 5.0-5.5: 12.5mg once daily. potassium >5.5: stop | |
|
Start 0.5mg, 0.25mg and 0,25 mg, each with 6 hours in between
Continue with 0.25mg once daily. Half dose with age above 70 or creatinin above 110 or with amiodarone use | |
| AT II blockers | ||
|
Start 4mg
3-5 weeks 8mg once daily. >7 weeks 16mg once daily. | |
|
Start 40mg twice daily
3-5 weeks 80mg twice daily. >7 weeks 160mg twice daily. | |
| Hydralazine and isosorbide dinitrate (H-ISDN) | ||
|
Start 25mg three times daily.
3-5 weeks 50mg three times daily. >7 weeks 75-100mg three times daily. | |
|
Start 20mg twice daily
3-5 weeks 40mg twice daily >7 weeks 80mg twice daily | |
Management of HF beyond medication
Device treatment
Prevention of sudden death is an important goal in HF because approximately half of the deaths occur suddenly, and many of these are related to ventricular arrhythmias. Implantable cardioverter-defibrillator (ICD) therapy is recommended in survivors of cardiac arrest , irrespective of EF, when life expectancy is >1 year. (Class I recommendation, level of evidence A).
In symptomatic HF patients (NYHA class II-III) with an EF ≤35% after more than 3 months of pharmacological treatment and a life expectancy >1 year, prophylactic ICD implantation is recommended in patients with ischemic etiology (Class I recommendation, level of evidence A) and non-ischemic etiology (Class I recommendation, level of evidence B).
Cardiac resynchronization therapy (CRT) is indicated in patients with symptomatic heart failure with one type and severity of ventricular conduction delay (LBBB, QRS ≥120 msec), and preferably in patients with sinus rhythm. The responder rate (improvement of at least 5% EF) is about 70%. Recommendation for use of this therapy differs according to heart rhythm, NYHA class, QRS duration and morphology, and LVEF. This is depicted in the Figure 8.
Timing of ICD implantation
Figure 5 offers recommendations to which patients should receive ICD treatment. In this flowchart, the timing of the placement has not been defined completely. In most patients, it should be safe to wait for their ICD whilst receiving (pharmacological) treatment as events typically occur after 6-12 months.[11] An exception to this rule is the group of high risk patients (i.e. patients with major myocardial infarction (MI), who have extensive fibrosis on the MRI or NSVT despite optimal pharmacological treatment); an ICD implantation should not be postponed too long in these patients. Early (within 40 days after event) ICD placement after an acute myocardial infarction has not been shown to reduce mortality, because the patients most at risk of sudden death are also the patients most at risk of death due to heart failure.[12][13][14] For this reason, prophylactic ICD treatment is recommended only after 40 days in post-infarct patients who have an EF < 35%. For non-ischemic heart failure patients, three months is considered a safe waiting time for an ICD. There are, however, also higher risk patients among this group, and a decision should be made for each patient on an individual basis.[15]
Heart transplantation and Left Ventricular Assist Devices
When a patient has severe and progressive HF, his or her prognosis is grim. Considering the paucity of donor hearts, the waiting list for heart transplantation may be long and early consideration of heart transplantation is part of the treatment strategy in HF. Average 2-year survival rates after cardiac transplantation are approximately 80%. A patient in NYHA class III should be evaluated with an exercise test for maximal oxygen uptake, in order to consider further steps. Indication for heart transplantation includes a VO2max < 14 ml/min/kg.[16]
Exclusion criteria are pulmonary hypertension (risk of immediate RV donor failure), severe comorbidity, and diabetes mellitus with organ damage. Left Ventricular Assist Devices are more commonly used as a bridge to transplantation, when the patient in on a waiting list. They have evolved from pulsatile to continuous flow pumps, with less complications and a longer durability. Often Left Ventricular Assist Devices become destination therapy.
Management of HF patients with preserved LVEF (HFPEF)
To date, no evidence exists of any treatment reducing morbidity or mortality in this patient group. With the aim of controlling water and sodium retention and to decrease breathlessness and edema, diuretics are prescribed to HFPEF patients. Furthermore, ACE-I, Angiotensin II blockers and/or beta blockers may be considered. The CHARM trial including 3023 HF patients with preserved EF, showed angiotensin II blockade (candesartan) to have a moderate effect on hospital admission but showed no effect on the risk of cardiovascular death.[17]
Prognosis
The life expectancy of a patient with heart failure is determined by age, NYHA class, LVEF, normal level of sodium, systolic blood pressure, use of medication and use of ICD or CRT-D (Seattle Heart failure score). The mean yearly annual mortality is approximately 10%, varying from <6% per year when a normal LVEF is identified, to > 14% per year with an EF of <15%. Trials with medication illustrate that the (short term) benefit of medication is highest when the NYHA class is higher (Figure 9).[18]
References
-
Withering W., Keys TE, An account of the foxglove and some of its medical uses, with practical remarks on dropsy, and other diseases, Classics of Cardiology. Volume I. New York, NY: Henry Schuman, Dover Publications; 1941: 231–252.
-
Kannel WB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for Estimating Risk of Heart Failure, Arch Intern Med. 1999;159:1197-204
-
McMurray JJV et al., ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012, European Heart Journal (2012; 33;1787–1847)
-
McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJ, Dargie HJ. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997 Sep 20;350(9081):829-33
-
Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003 Aug 26;108(8):977-82. Epub 2003 Aug 11.
-
Holubarsch C et al, Existence of the Frank-Starling Mechanism in the Failing Human Heart Investigations on the Organ, Tissue, and Sarcomere Levels. Circulation. 1996 Aug 15;94(4):683-9.
-
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. New England Journal of Medicine. 1971; 23;285(26):1441-6.
-
Garg R, Yusuf S, Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Journal of the American Medical Association. 1995;273:1450-6
-
Foody JM, Farrell MH, Krumholz MH. ß-Blocker Therapy in Heart Failure. Scientific Review. Journal of the American Medical Association. 2002;287(7):883-889.
-
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. New England Journal of Medicine .1999;341:709-17
-
Moss AJ, Zareba W, Hall J, Klein H, Wilbur DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. New England Journal of Medicine. 2002;346:877–883.
-
Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. New England Journal of Medicine.2004;351:2481–2488
-
Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, WojciechowskiD, Kornacewich-Jach Z, Sredniawa B, Lupkovics G, Hofgartner F, Lubinski A, Rosenqvist M, Habets A, Wegscheider K, Senges J. Defibrillator implantation early after myocardial infarction. New England Journal of Medicine. 2009;361:1427–1436
-
Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, Burke M, MossAJ . Time-dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–1084.
-
Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with non-ischemic dilated cardiomyopathy. England Journal of Medicine. 2004;350:2151–2158
-
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786
-
Yusuf S. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. The Lancet. Sept 2003 362;9386:777-781
-
Cleland JGF, Andrew L, Clark AL. Delivering the cumulative benefits of triple therapy to improve outcomes in heart failure. Journal of the American College of Cardiology. 2003;42(7)
-
Mosterd A. Clinical epidemiology in heart failure. Heart 2007;93:1137-1146
-
McMurray JJ. Clinical practice. Systolic heart failure. New England Journal of Medicine. 2010;362: 228-238