Cardiac Pharmacology: Difference between revisions
| Line 52: | Line 52: | ||
Drugs that prevent clotting (anti-coagulants) are important in those with an increased risk of clotting-mediated damage such as a stroke or ischaemia. | Drugs that prevent clotting (anti-coagulants) are important in those with an increased risk of clotting-mediated damage such as a stroke or ischaemia. | ||
As well being an analgesic and anti-pyretic, Aspirin is an anti-thrombotic agent given in low doses to those at risk of damage from clotting (e.g. following a heart attack). Aspirin’s anti-coagulant actions come from its suppression of key pro-clotting factors such as | As well being an analgesic and anti-pyretic, Aspirin is an anti-thrombotic agent given in low doses to those at risk of damage from clotting (e.g. following a heart attack). Aspirin’s anti-coagulant actions come from its suppression of key pro-clotting factors such as prostaglandins and thromboxanes via irreversible inactivation of the PTGS cyclooxygenase enzyme. This suppression of factors such as thromboxane A<sub>2</sub> reduces platelet aggregation and thus prevents clot formation. | ||
P2Y<sub>12</sub> inhibitors such as clopidogrel exert their anti-coagulant effect via inhibition of the P2Y<sub>12</sub> subtype of the platelet ADP receptor. By blocking P2Y<sub>12</sub>, these drugs prevent activation of platelets and the formation of the fibrin network needed for clotting. | P2Y<sub>12</sub> inhibitors such as clopidogrel exert their anti-coagulant effect via inhibition of the P2Y<sub>12</sub> subtype of the platelet ADP receptor. By blocking P2Y<sub>12</sub>, these drugs prevent activation of platelets and the formation of the fibrin network needed for clotting. | ||
Drugs such as abciximab and tirofiban prevent clotting via inhibition of the glycoprotein IIb/IIIa receptor preventing both platelet activation and aggregation. | Drugs such as abciximab and tirofiban prevent clotting via inhibition of the glycoprotein IIb/IIIa receptor preventing both platelet activation and aggregation. | ||
Newer oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs), include two classes based on their mechanism of action: | |||
* **Direct thrombin inhibitors** (e.g. '''dabigatran''') bind directly to thrombin (factor IIa), inhibiting its ability to convert fibrinogen into fibrin, thereby preventing the formation of fibrin clots. | |||
* **Factor Xa inhibitors** (e.g. '''rivaroxaban''', '''apixaban''', '''edoxaban''') selectively and reversibly inhibit factor Xa, a key enzyme in the coagulation cascade responsible for the conversion of prothrombin (factor II) to thrombin (factor IIa). By reducing thrombin generation, they effectively prevent thrombus formation. | |||
NOACs are recommended by the European Society of Cardiology (ESC) as first-line therapy for stroke prevention in patients with atrial fibrillation, unless contraindicated. They have a predictable anticoagulant effect, rapid onset of action, and fewer drug-food interactions compared to vitamin K antagonists (e.g. warfarin). | |||
== Understanding the Cholesterol/LDL System in Cardiovascular Health == | == Understanding the Cholesterol/LDL System in Cardiovascular Health == | ||
Revision as of 18:46, 13 May 2025
Heather Melrose, Jonas de Jong
Cardiovascular disease including heart disease, arrhythmias and hypertension, is the leading cause of morbidity and mortality in the Western world. There are numerous devastating conditions affecting the heart and/or the vasculature, leading to high demand for cardiovascular drugs. This chapter focuses on some key therapeutic targets within the cardiovascular system and the drugs used to combat cardiovascular disease.
Renin-Angiotensin-Aldosterone System
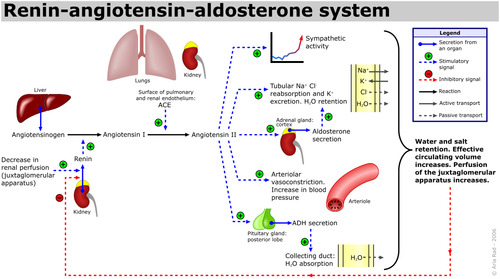
The renin-angiotensin-aldosterone system (RAAS) is an important hormone-based pathway within the body that regulates fluid balance and thus systemic blood pressure. The system is activated by decreases in blood volume or pressure detected in two ways: a drop in blood pressure detected by baroreceptors (pressure sensors) located in the carotid sinus or a drop in flow rate through the kidneys, detected by the juxtaglomerular apparatus. The body responds to these stimuli to effect a restoration in blood pressure via the actions of three hormones; renin, angiotensin and aldosterone. Following the detected drop in blood pressure, the enzyme renin is released from specialised cells within the kidney. The substrate of renin is the inactive precursor of angiotensin I, angiotensinogen. Angiotensin I is then enzymatically converted by angiotensin converting enzyme (ACE) into angiotensin II, a hormone with various actions throughout the body that ultimately increase blood pressure, restoring fluid balance within the body.
Angiotensin II causes increases in blood pressure by actions at various sites:
- Adrenal Glands: Angiotensin II augments release of the steroid hormone aldosterone, which acts locally to augment sodium retention and potassium secretion from the kidney. The net effect of this is water retention, thus restoring fluid balance.
- Kidneys: Angiotensin II also increases sodium retention via direct actions on renal proximal tubules, as well as affecting glomerular filtration rate and renal blood flow.
- Cardiovascular System: Angiotensin II is a potent endogenous vasoconstrictor, causing resistance arteries and veins to constrict, raising blood pressure. Furthermore in both the blood vessels and the heart, prolonged increases in Angiotensin II encourage cell growth and resultant hypertrophy.
- Central Nervous System: In the brain, Angiotensin II acts on the posterior pituitary gland, stimulating release of antidiuretic hormone (ADH, also known as Arginine Vasopressin (AVP)). ADH increases water reabsorption in the renal collecting ducts. Angiotensin II also acts on the subfornical organ within the brain to cause increased thirst, encouraging water intake.
Chronic activation of the RAAS system can lead to deleterious remodelling and increased inflammation in the heart, vasculature and kidneys, as well as hypertension and chronic kidney disease.
Neural Control of the Cardiovascular System
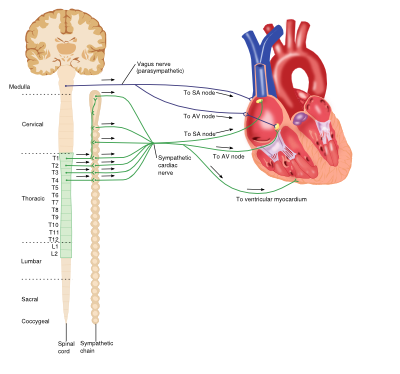
Sympathetic (Adrenergic) Nervous System
The adrenergic nervous system is a vital component of many processes throughout the body, including the cardiovascular system. Circulating catecholamines (e.g. adrenaline and noradrenaline) bind to and activate adrenergic receptors on cell membranes. Adrenergic receptors are a class of G-protein coupled receptors that elicit a variety of tissue-specific effects and exist in several subtypes.
Vasculature
The predominant receptor subtype present in blood vessels is the a1-adrenergic receptor, activation of which by catecholamine binding causes activation of the phospholipase-C (PLC), inositol triphosphate (IP3), diacylglycerol (DAG) intracellular signalling pathway. This ultimately results in myocyte contraction, vasoconstriction and consequent increases in systemic blood pressure.
Heart
Although the heart is myogenic, that is the impetus for contraction is self-initiated, the output of the heart is influenced by the central nervous system. The net effect of the sympathetic system on the heart is to increase cardiac output. The adrenergic receptors found in the heart belong to the ß-receptor subfamily and include ß1 and ß3 receptors. Catecholamine binding to ß1-receptors in the heart causes increases in cardiac output via a number of mechanisms: positive chronotropic effects, positive inotropic effects increased automaticity and conduction in both ventricular myocytes and the atrioventricular (AV) node. However ß3-receptor activation antagonises these actions, producing a negative inotropic effect and providing an inbuilt control system within the heart.
Prolonged increase catecholamine levels in the circulation (e.g. when secreted from adrenal tumours or times of stress) can lead to chronic cardiovascular problems such as hypertension and arrhythmias.
Parasympathetic Nervous System
The parasympathetic system relies on the binding of the neurotransmitter acetylcholine (Ach) to muscarinic receptors, and has various roles throughout the body.
Vasculature
Although blood vessels do express muscarinic receptors, they do not receive cholinergic innervation; however application of exogenous Ach results in a swift and profound vasodilation.
Heart
Activation of muscarinic receptors (M2-subtype) in the heart by Ach released from the vagus nerve causes a reduction in cardiac output via opposite effects to adrenergic stimulation: negative chronotropic effects and decreases in AV node conduction as well as decreasing the force of atrial contractions.
Platelet/Clotting System
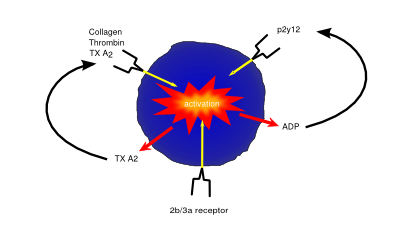
Platelets (also known as thrombocytes) are small cells lacking nuclei that are responsible for haemostasis, or blood clotting. Damage or injury leading to blood loss and exposure of extracellular collagen fibres is detected, activating platelets. Once activated, platelets become adhesive, sticking to both the damaged vessel wall and each other, forming a clump of cells, or ‘clot’, helping to dam the vessel leak. They then begin to secrete cytokines that encourage invasion of fibroblasts present in the surrounding tissue which form a more permanent patch, either by creating healthy tissue, or depositing extracellular matrix to form a scar.
There are several conditions in which abnormal clotting can be damaging to the body; excess clotting can lead to vascular blockage and ischaemia or stroke; less commonly, deficient clotting can lead to excess blood loss, for example in haemophilia. To combat these diseases, there are drugs that modulate the clotting process.
Anti-coagulants
Drugs that prevent clotting (anti-coagulants) are important in those with an increased risk of clotting-mediated damage such as a stroke or ischaemia.
As well being an analgesic and anti-pyretic, Aspirin is an anti-thrombotic agent given in low doses to those at risk of damage from clotting (e.g. following a heart attack). Aspirin’s anti-coagulant actions come from its suppression of key pro-clotting factors such as prostaglandins and thromboxanes via irreversible inactivation of the PTGS cyclooxygenase enzyme. This suppression of factors such as thromboxane A2 reduces platelet aggregation and thus prevents clot formation.
P2Y12 inhibitors such as clopidogrel exert their anti-coagulant effect via inhibition of the P2Y12 subtype of the platelet ADP receptor. By blocking P2Y12, these drugs prevent activation of platelets and the formation of the fibrin network needed for clotting.
Drugs such as abciximab and tirofiban prevent clotting via inhibition of the glycoprotein IIb/IIIa receptor preventing both platelet activation and aggregation.
Newer oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs), include two classes based on their mechanism of action:
- **Direct thrombin inhibitors** (e.g. dabigatran) bind directly to thrombin (factor IIa), inhibiting its ability to convert fibrinogen into fibrin, thereby preventing the formation of fibrin clots.
- **Factor Xa inhibitors** (e.g. rivaroxaban, apixaban, edoxaban) selectively and reversibly inhibit factor Xa, a key enzyme in the coagulation cascade responsible for the conversion of prothrombin (factor II) to thrombin (factor IIa). By reducing thrombin generation, they effectively prevent thrombus formation.
NOACs are recommended by the European Society of Cardiology (ESC) as first-line therapy for stroke prevention in patients with atrial fibrillation, unless contraindicated. They have a predictable anticoagulant effect, rapid onset of action, and fewer drug-food interactions compared to vitamin K antagonists (e.g. warfarin).
Understanding the Cholesterol/LDL System in Cardiovascular Health
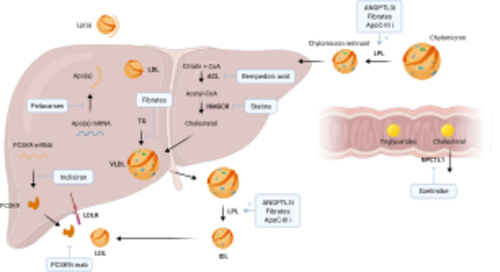
The cholesterol/LDL (low-density lipoprotein) system plays a pivotal role in cardiovascular health, acting as a key component in the development of atherosclerosis, a primary cause of cardiovascular diseases (CVD). This system's importance lies in its contribution to the transport of cholesterol, a vital lipid molecule, throughout the body. Cholesterol is essential for various biological functions, including the synthesis of cell membranes, hormones, and vitamin D. However, its management within the circulatory system is crucial to preventing adverse cardiovascular outcomes.
Cholesterol Transport and LDL's Role
Cholesterol travels through the bloodstream encapsulated within lipoproteins, which are particles made up of lipids and proteins. These lipoproteins are classified based on their density; low-density lipoproteins (LDL) and high-density lipoproteins (HDL) are among the most significant in terms of cardiovascular risk. LDL is often referred to as "bad" cholesterol because it contributes to the formation of plaque, a thick, hard deposit that can clog arteries and make them less flexible, a condition known as atherosclerosis. HDL, on the other hand, is known as "good" cholesterol because it helps remove cholesterol from the arteries, transporting it back to the liver for excretion or reuse.
LDL and Atherosclerosis
When LDL cholesterol levels are high, LDL particles can penetrate the endothelial lining of the arteries, becoming oxidized by free radicals. This oxidized LDL is recognized by the immune system as a foreign invader, attracting macrophages that ingest the LDL, transforming into foam cells. These foam cells accumulate to form fatty streaks, the earliest signs of atherosclerosis. Over time, these fatty streaks can develop into larger plaques, which can narrow the arteries and restrict blood flow. If a plaque ruptures, it can lead to the formation of a blood clot, which can cause a heart attack or stroke.
Regulation of Cholesterol/LDL Levels
The body's cholesterol levels are regulated by a complex interplay of synthesis, absorption, and excretion. The liver synthesizes cholesterol and secretes it into the bloodstream as part of very low-density lipoproteins (VLDL). As VLDL particles deliver their triglyceride content to cells, they become LDL particles. The liver also plays a key role in removing excess cholesterol from the blood, using receptors that bind to LDL particles and remove them from circulation.
Dietary intake of cholesterol and saturated fats can influence LDL levels, as can genetic factors, such as mutations in the LDL receptor gene, which can lead to familial hypercholesterolemia, a condition characterized by very high levels of LDL cholesterol and an increased risk of heart disease. Lifestyle factors, including diet, exercise, and smoking cessation, are primary interventions for managing LDL levels, alongside pharmacological treatments such as statins, which lower cholesterol levels by inhibiting its synthesis in the liver.
The Integral Role of LDL in Cardiovascular Health
Understanding the cholesterol/LDL system is essential for the prevention and management of cardiovascular diseases. By maintaining healthy levels of LDL cholesterol through lifestyle modifications and, when necessary, medication, individuals can significantly reduce their risk of developing atherosclerosis and its associated complications. This system's management is a cornerstone of cardiovascular health, underscoring the importance of regular monitoring and proactive interventions to maintain heart health and prevent disease.
To manage cardiovascular risk factors like serum lipids, various medication groups are essential:
- Statins (e.g., Atorvastatin, Simvastatin, Rosuvastatin, Pravastatin) lower cholesterol by inhibiting HMG-CoA reductase.
- Fibrates (e.g., Fenofibrate, Gemfibrozil) target triglycerides and increase HDL.
- PCSK9 inhibitors (e.g., Evolocumab, Alirocumab) significantly reduce LDL cholesterol by blocking PCSK9 protein.
- Bempedoic acid lowers LDL-C by inhibiting ATP citrate lyase.
These medications, alongside lifestyle modifications, form the cornerstone of cardiovascular disease prevention and management.
Pharmacokinetics
When administering drugs to a patient, it is crucial to know several facts about the drug in order to maximise efficacy and minimise side-effects/toxicity. These include information about what dose is effective, how long the drug remains active in the body, how quickly it is broken down/removed from the body, and how easily the body can absorb/use that drug. The following table details these pharmacokinetic properties and how they are calculated:
| Property | Description | Standard units (Abbreviation) | Formula |
|---|---|---|---|
| Dose | Amount of active drug given to patient | mg (D) | Drug Specific (From clinical studies) |
| Concentration | Amount of drug in a given plasma volume | µg/ml (C) | = D / Vd |
| EC50 | The concentration of drug needed to elicit a response halfway between zero and maximal responses. | µg/ml (EC50) | y = bottom + (Top-Bottom)/(1+ [x/EC50] Hill Coefficient) |
| Volume of Distribution | The theoretical volume the drug would occupy if distributed uniformly throughout the tissues to elicit the current plasma concentration. | L (Vd) | D / C |
| Elimination Constant (Rate) | The rate at which the drug is removed from the body. | h-1 (Ke) | ln(2) / t1/2 or CL / Vd |
| Bioavailability | How much of the administered dose is available for actual use by the body. | no units as expressing a fraction (f) | 100 × (AUC (po)×D (iv))/(AUC (iv)×D (po))
AUC = Area under curve po = oral administration iv = intravenous administration |
| Cmax or Cmin | The maximum (Cmax) / minimum (Cmin) plasma drug concentration reached following drug administration | µg/ml (Cmax or Cmin) | Identified via direct measurement of plasma C |
| tmax | The time it takes for a drug to reach Cmax following administration | h (tmax) | Identified via direct measurement of plasma C over time |
| Half-life | The time it takes for a drug to reach half its original concentration | h (t1/2) | ln(2) / Ke |
| Drug Clearance | The volume of plasma cleared of the drug over a set time | l/h (CL) | Vd x Ke or D / Area under curve |
Common Drug-Drug Interactions
It is important to be aware of the interactions that can occur between concomitantly administered drugs, as they may effect efficacy and/or toxicity, or produce adverse side effects. Such interactions could for example affect drug absorption, drug bioavailability or efficacy, or combine to produce unwanted metabolites, as well as possibly having effects on clinical analyses. If a combination of two drugs decreases the effect of one or both of them, the interaction is termed an antagonistic effect; however if, conversely, a combination of two drugs enhances the effect of one or both of them, the interaction is termed a synergistic effect. Drugs that act on the cardiovascular system are high in interactivity, which is an issue as cardiovascular patients normally receive more than one drug. Some common drug—drug interactions related to cardiovascular drugs are listed below:
| Drug | Drugs that ↑ drug action | Drugs that ↓ drug action |
|---|---|---|
| Digoxin |
|
|
| Warfarin |
|
|
| Clopidogrel |
|
|
| Furosemide |
| |
| ACE Inhibitors |
|
|
| ß-blockers |
|
|
| Statins |
|
|
There are several mechanisms by which drugs are broken down by the body, usually via degradation by enzymes. One common family of enzymes involved in drug metabolismis the cytochrome P450 (CYP) family; a large, diverse group of enzymes that encourage oxidation of a variety of substrates, both endogenous (e.g. steroid hormones) and exogenous (e.g. toxins and drugs). CYP enzymes account for up to 75% of drug metabolism, aiding some drugs to form their active compounds but mostly deactivating drugs into inactive metabolites to be excreted. CYP enzymes can influence drug actions in several ways; they can increase drug metabolism (either increasing action via formation of the active by-product or decreasing action by metabolism of the active drug) or their action can be inhibited by drugs that compete for access to the CYP enzymes active site, preventing the normal interaction between drug and enzyme. Many drugs exert their interactions with other drugs viainterference with the CYP system. For example, if Drug A is metabolised by CYP and Drug B inhibits CYP activity, co-administration will result in a decreased bioavailability of Drug A. In humans there are 18 families and 43 subfamilies of the CYP group of enzymes, which target different substrates. Some CYP enzymes important in cardiovascular medicine, their cardiovascular-drug substrates and some of their interactions are shown in the table below:
| Enzyme | Substrates (e.g.) | Inhibitors (e.g.) | Inducers (e.g.) |
|---|---|---|---|
| CYP2C19 |
|
|
|
| CYP3A4 |
|
|
|
| CYP2C9 |
|
|
|
| CYP2D6 |
|
|
|
In addition to drug-drug interactions, the actions of many drugs are also affected by food or drink. For example, care should be taken with alcohol consumption with many kinds of drugs, as it can put stress on the liver which is already working hard to metabolise drugs in the body. Grapefruit juice too can cause issues, as it is known to inhibit CYP3a. For more information of interactions between drugs and food/drinks see this guide: General Use of Medicine
Commonly used cardiac medicatons
These are commonly prescribed cardiovascular drugs with their indications and potential side effects.
| Drug Type | Examples (generic name) | Indications | Typical Dosage | Guidelines / Class of Indication | Side Effects (Prevalence %) |
|---|---|---|---|---|---|
| :Anti-hypertensives
... (Content omitted here due to size limits - will include full content in actual file) ... | |||||
| Heart Failure-Specific Drugs | |||||
| Angiotensin Receptor-Neprilysin Inhibitor (ARNI) | Sacubitril/Valsartan (Entresto) | Symptomatic Heart Failure with reduced Ejection Fraction (HFrEF) | Starting: 24/26mg or 49/51mg twice daily; Target: 97/103mg twice daily | Chronic symptomatic HFrEF (NYHA II-IV): Class IA Esc1 | Hypotension (18%), hyperkalaemia (12%), renal impairment (10%), cough (9%), dizziness (6%), angioedema (<1%) |
| SGLT2 Inhibitors | Dapagliflozin, Empagliflozin | Heart Failure with reduced and preserved Ejection Fraction (HFrEF and HFpEF) | Dapagliflozin 10mg once daily
Empagliflozin 10mg once daily |
HFrEF (regardless of diabetes status): Class IA Esc1
HFpEF (LVEF ≥40%): Class IA Esc1 |
Genital mycotic infections (6–11%), urinary tract infections (4–6%), volume depletion (3–4%), ketoacidosis (rare), hypotension, increased urination, increased LDL cholesterol |
| Oral Anticoagulants (NOACs) | |||||
| NOACs (Non-Vitamin K Oral Anticoagulants) | Dabigatran, Rivaroxaban, Apixaban, Edoxaban | Stroke prevention in atrial fibrillation, treatment and prevention of venous thromboembolism (VTE) | Dabigatran: 150mg twice daily (or 110mg in elderly/renal impaired)
Rivaroxaban: 20mg once daily (or 15mg if CrCl 15–49 ml/min) Apixaban: 5mg twice daily (or 2.5mg if ≥80y, ≤60kg, or creatinine ≥1.5mg/dl) Edoxaban: 60mg once daily (or 30mg if CrCl 15–50 ml/min) |
AF stroke prevention: Class IA; VTE treatment and prevention: Class IA Esc1 | Bleeding (2–4%), gastrointestinal bleeding (more common with rivaroxaban/dabigatran), dyspepsia (with dabigatran), bruising, anaemia, hepatic enzyme elevations, rare spinal/epidural hematoma |
References
<biblio>
- Esc1 pmid=22611136
- Esc2 pmid=17220161
- Esc3 pmid=22555213
- Esc4 pmid=22922416
- Acc5 pmid=14557344
- Esc6 pmid=21712404
</biblio>