Cardiac Pharmacology: Difference between revisions
No edit summary |
|||
| (61 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
''Heather Melrose, Jonas de Jong'' | |||
__TOC__ | |||
Cardiovascular disease including heart disease, arrhythmias and hypertension, is the leading cause of morbidity and mortality in the Western world. There are numerous devastating conditions affecting the heart and/or the vasculature, leading to high demand for cardiovascular drugs. This chapter focuses on some key therapeutic targets within the cardiovascular system and the drugs used to combat cardiovascular disease. | Cardiovascular disease including heart disease, arrhythmias and hypertension, is the leading cause of morbidity and mortality in the Western world. There are numerous devastating conditions affecting the heart and/or the vasculature, leading to high demand for cardiovascular drugs. This chapter focuses on some key therapeutic targets within the cardiovascular system and the drugs used to combat cardiovascular disease. | ||
==Renin-Angiotensin-Aldosterone System== | ==Renin-Angiotensin-Aldosterone System== | ||
[[Image:Renin-angiotensin-aldosterone_system.png|thumb|right|500px|RAAS schematic]] | |||
The renin-angiotensin-aldosterone system (RAAS) is an important hormone-based pathway within the body that regulates fluid balance and thus systemic blood pressure. The system is activated by decreases in blood volume or pressure detected in two ways: a drop in blood pressure detected by baroreceptors (pressure sensors) located in the carotid sinus or a drop in flow rate through the kidneys, detected by the juxtaglomerular apparatus. The body responds to these stimuli to effect a restoration in blood pressure via the actions of three hormones; renin, angiotensin and aldosterone. Following the detected drop in blood pressure, the enzyme renin is released from specialised cells within the kidney. The substrate of renin is the inactive precursor of angiotensin I, angiotensinogen. Angiotensin I is then enzymatically converted by angiotensin converting enzyme (ACE) into angiotensin II, a hormone with various actions throughout the body that ultimately increase blood pressure, restoring fluid balance within the body. | The renin-angiotensin-aldosterone system (RAAS) is an important hormone-based pathway within the body that regulates fluid balance and thus systemic blood pressure. The system is activated by decreases in blood volume or pressure detected in two ways: a drop in blood pressure detected by baroreceptors (pressure sensors) located in the carotid sinus or a drop in flow rate through the kidneys, detected by the juxtaglomerular apparatus. The body responds to these stimuli to effect a restoration in blood pressure via the actions of three hormones; renin, angiotensin and aldosterone. Following the detected drop in blood pressure, the enzyme renin is released from specialised cells within the kidney. The substrate of renin is the inactive precursor of angiotensin I, angiotensinogen. Angiotensin I is then enzymatically converted by angiotensin converting enzyme (ACE) into angiotensin II, a hormone with various actions throughout the body that ultimately increase blood pressure, restoring fluid balance within the body. | ||
| Line 19: | Line 22: | ||
==Neural Control of the Cardiovascular System== | ==Neural Control of the Cardiovascular System== | ||
[[File:Sympathic_parasympathic.svg|thumb|400px|Interaction between the sympathic and parasympathic nervous system and the heart]] | |||
===Sympathetic (Adrenergic) Nervous System=== | ===Sympathetic (Adrenergic) Nervous System=== | ||
The adrenergic nervous system is a vital component of many processes throughout the body, including the cardiovascular system. Circulating catecholamines (e.g. adrenaline and noradrenaline) bind to and activate adrenergic receptors on cell membranes. Adrenergic receptors are a class of G-protein coupled receptors that elicit a variety of tissue-specific effects and exist in several subtypes. | The adrenergic nervous system is a vital component of many processes throughout the body, including the cardiovascular system. Circulating catecholamines (e.g. adrenaline and noradrenaline) bind to and activate adrenergic receptors on cell membranes. Adrenergic receptors are a class of G-protein coupled receptors that elicit a variety of tissue-specific effects and exist in several subtypes. | ||
====Vasculature==== | ====Vasculature==== | ||
The predominant receptor subtype present in blood vessels is the | The predominant receptor subtype present in blood vessels is the a1-adrenergic receptor, activation of which by catecholamine binding causes activation of the phospholipase-C (PLC), inositol triphosphate (IP3), diacylglycerol (DAG) intracellular signalling pathway. This ultimately results in myocyte contraction, vasoconstriction and consequent increases in systemic blood pressure. | ||
====Heart==== | ====Heart==== | ||
Although the heart is myogenic, that is the impetus for contraction is self-initiated, the output of the heart is influenced by the central nervous system. The net effect of the sympathetic system on the heart is to increase cardiac output. The adrenergic receptors found in the heart belong to the | Although the heart is myogenic, that is the impetus for contraction is self-initiated, the output of the heart is influenced by the central nervous system. The net effect of the sympathetic system on the heart is to increase cardiac output. The adrenergic receptors found in the heart belong to the ß-receptor subfamily and include ß1 and ß3 receptors. Catecholamine binding to ß1-receptors in the heart causes increases in cardiac output via a number of mechanisms: positive chronotropic effects, positive inotropic effects increased automaticity and conduction in both ventricular myocytes and the atrioventricular (AV) node. However ß3-receptor activation antagonises these actions, producing a negative inotropic effect and providing an inbuilt control system within the heart. | ||
Prolonged increase catecholamine levels in the circulation (e.g. when secreted from adrenal tumours or times of stress) can lead to chronic cardiovascular problems such as hypertension and arrhythmias. | Prolonged increase catecholamine levels in the circulation (e.g. when secreted from adrenal tumours or times of stress) can lead to chronic cardiovascular problems such as hypertension and arrhythmias. | ||
| Line 40: | Line 44: | ||
==Platelet/Clotting System== | ==Platelet/Clotting System== | ||
[[File:Platelet_receptors.svg|thumb|400px|Platelet activation and inhibition operates through surface receptors on platelets. Feedback loops enhance platelet activation (e.g. ADP released by platelets increases platelet activation, through the ADP receptor)]] | |||
Platelets (also known as thrombocytes) are small cells lacking nuclei that are responsible for haemostasis, or blood clotting. Damage or injury leading to blood loss and exposure of extracellular collagen fibres is detected, activating platelets. Once activated, platelets become adhesive, sticking to both the damaged vessel wall and each other, forming a clump of cells, or ‘clot’, helping to dam the vessel leak. They then begin to secrete cytokines that encourage invasion of fibroblasts present in the surrounding tissue which form a more permanent patch, either by creating healthy tissue, or depositing extracellular matrix to form a scar. | Platelets (also known as thrombocytes) are small cells lacking nuclei that are responsible for haemostasis, or blood clotting. Damage or injury leading to blood loss and exposure of extracellular collagen fibres is detected, activating platelets. Once activated, platelets become adhesive, sticking to both the damaged vessel wall and each other, forming a clump of cells, or ‘clot’, helping to dam the vessel leak. They then begin to secrete cytokines that encourage invasion of fibroblasts present in the surrounding tissue which form a more permanent patch, either by creating healthy tissue, or depositing extracellular matrix to form a scar. | ||
| Line 47: | Line 52: | ||
Drugs that prevent clotting (anti-coagulants) are important in those with an increased risk of clotting-mediated damage such as a stroke or ischaemia. | Drugs that prevent clotting (anti-coagulants) are important in those with an increased risk of clotting-mediated damage such as a stroke or ischaemia. | ||
As well being an analgesic and anti-pyretic, Aspirin is an anti-thrombotic agent given in low doses to those at risk of damage from clotting (e.g. following a heart attack). Aspirin’s anti-coagulant actions come from its suppression of key pro-clotting factors such as | As well being an analgesic and anti-pyretic, Aspirin is an anti-thrombotic agent given in low doses to those at risk of damage from clotting (e.g. following a heart attack). Aspirin’s anti-coagulant actions come from its suppression of key pro-clotting factors such as prostaglandins and thromboxanes via irreversible inactivation of the PTGS cyclooxygenase enzyme. This suppression of factors such as thromboxane A<sub>2</sub> reduces platelet aggregation and thus prevents clot formation. | ||
P2Y<sub>12</sub> inhibitors such as clopidogrel exert their anti-coagulant effect via inhibition of the P2Y<sub>12</sub> subtype of the platelet ADP receptor. By blocking P2Y<sub>12</sub>, these drugs prevent activation of platelets and the formation of the fibrin network needed for clotting. | P2Y<sub>12</sub> inhibitors such as clopidogrel exert their anti-coagulant effect via inhibition of the P2Y<sub>12</sub> subtype of the platelet ADP receptor. By blocking P2Y<sub>12</sub>, these drugs prevent activation of platelets and the formation of the fibrin network needed for clotting. | ||
Drugs such as abciximab and tirofiban prevent clotting via inhibition of the glycoprotein IIb/IIIa receptor preventing both platelet activation and aggregation. | Drugs such as abciximab and tirofiban prevent clotting via inhibition of the glycoprotein IIb/IIIa receptor preventing both platelet activation and aggregation. | ||
== | Newer oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs), include two classes based on their mechanism of action: | ||
* **Direct thrombin inhibitors** (e.g. '''dabigatran''') bind directly to thrombin (factor IIa), inhibiting its ability to convert fibrinogen into fibrin, thereby preventing the formation of fibrin clots. | |||
* **Factor Xa inhibitors** (e.g. '''rivaroxaban''', '''apixaban''', '''edoxaban''') selectively and reversibly inhibit factor Xa, a key enzyme in the coagulation cascade responsible for the conversion of prothrombin (factor II) to thrombin (factor IIa). By reducing thrombin generation, they effectively prevent thrombus formation. | |||
NOACs are recommended by the European Society of Cardiology (ESC) as first-line therapy for stroke prevention in patients with atrial fibrillation, unless contraindicated. They have a predictable anticoagulant effect, rapid onset of action, and fewer drug-food interactions compared to vitamin K antagonists (e.g. warfarin). | |||
== Understanding the Cholesterol/LDL System in Cardiovascular Health == | |||
[[File:Lipid metabolism2.svg|thumb|Lipid mechanisms of action of common drugs|500x500px]] | |||
The cholesterol/LDL (low-density lipoprotein) system plays a pivotal role in cardiovascular health, acting as a key component in the development of atherosclerosis, a primary cause of cardiovascular diseases (CVD). This system's importance lies in its contribution to the transport of cholesterol, a vital lipid molecule, throughout the body. Cholesterol is essential for various biological functions, including the synthesis of cell membranes, hormones, and vitamin D. However, its management within the circulatory system is crucial to preventing adverse cardiovascular outcomes. | |||
==== Cholesterol Transport and LDL's Role ==== | |||
Cholesterol travels through the bloodstream encapsulated within lipoproteins, which are particles made up of lipids and proteins. These lipoproteins are classified based on their density; low-density lipoproteins (LDL) and high-density lipoproteins (HDL) are among the most significant in terms of cardiovascular risk. LDL is often referred to as "bad" cholesterol because it contributes to the formation of plaque, a thick, hard deposit that can clog arteries and make them less flexible, a condition known as atherosclerosis. HDL, on the other hand, is known as "good" cholesterol because it helps remove cholesterol from the arteries, transporting it back to the liver for excretion or reuse. | |||
==== LDL and Atherosclerosis ==== | |||
When LDL cholesterol levels are high, LDL particles can penetrate the endothelial lining of the arteries, becoming oxidized by free radicals. This oxidized LDL is recognized by the immune system as a foreign invader, attracting macrophages that ingest the LDL, transforming into foam cells. These foam cells accumulate to form fatty streaks, the earliest signs of atherosclerosis. Over time, these fatty streaks can develop into larger plaques, which can narrow the arteries and restrict blood flow. If a plaque ruptures, it can lead to the formation of a blood clot, which can cause a heart attack or stroke. | |||
==== Regulation of Cholesterol/LDL Levels ==== | |||
The body's cholesterol levels are regulated by a complex interplay of synthesis, absorption, and excretion. The liver synthesizes cholesterol and secretes it into the bloodstream as part of very low-density lipoproteins (VLDL). As VLDL particles deliver their triglyceride content to cells, they become LDL particles. The liver also plays a key role in removing excess cholesterol from the blood, using receptors that bind to LDL particles and remove them from circulation. | |||
Dietary intake of cholesterol and saturated fats can influence LDL levels, as can genetic factors, such as mutations in the LDL receptor gene, which can lead to familial hypercholesterolemia, a condition characterized by very high levels of LDL cholesterol and an increased risk of heart disease. Lifestyle factors, including diet, exercise, and smoking cessation, are primary interventions for managing LDL levels, alongside pharmacological treatments such as statins, which lower cholesterol levels by inhibiting its synthesis in the liver. | |||
==== The Integral Role of LDL in Cardiovascular Health ==== | |||
Understanding the cholesterol/LDL system is essential for the prevention and management of cardiovascular diseases. By maintaining healthy levels of LDL cholesterol through lifestyle modifications and, when necessary, medication, individuals can significantly reduce their risk of developing atherosclerosis and its associated complications. This system's management is a cornerstone of cardiovascular health, underscoring the importance of regular monitoring and proactive interventions to maintain heart health and prevent disease. | |||
To manage cardiovascular risk factors like serum lipids, various medication groups are essential: | |||
* '''Statins''' (e.g., Atorvastatin, Simvastatin, Rosuvastatin, Pravastatin) lower cholesterol by inhibiting HMG-CoA reductase. | |||
* '''Fibrates''' (e.g., Fenofibrate, Gemfibrozil) target triglycerides and increase HDL. | |||
* '''PCSK9 inhibitors''' (e.g., Evolocumab, Alirocumab) significantly reduce LDL cholesterol by blocking PCSK9 protein. | |||
* '''Bempedoic acid''' lowers LDL-C by inhibiting ATP citrate lyase. | |||
These medications, alongside lifestyle modifications, form the cornerstone of cardiovascular disease prevention and management. | |||
==Pharmacokinetics== | |||
When administering drugs to a patient, it is crucial to know several facts about the drug in order to maximise efficacy and minimise side-effects/toxicity. These include information about what dose is effective, how long the drug remains active in the body, how quickly it is broken down/removed from the body, and how easily the body can absorb/use that drug. The following table details these pharmacokinetic properties and how they are calculated: | |||
{| class="wikitable" border="0" cellpadding="0" cellspacing="0" | |||
|- | |||
!Property | |||
!Description | |||
!Standard units (Abbreviation) | |||
!Formula | |||
|- | |||
!Dose | |||
|Amount of active drug given to patient | |||
|align="center"|mg (D) | |||
|Drug Specific (From clinical studies) | |||
|- | |||
!Concentration | |||
|Amount of drug in a given plasma volume | |||
|align="center"|µg/ml (C) | |||
|align="center"|= D / Vd | |||
|- | |||
!EC<sub>50</sub> | |||
|The concentration of drug needed to elicit a response halfway between zero and maximal responses. | |||
|align="center"|µg/ml (EC<sub>50</sub>) | |||
|align="center"|y = bottom + (Top-Bottom)/(1+ [x/EC50] Hill Coefficient) | |||
|- | |||
!Volume of Distribution | |||
|The theoretical volume the drug would occupy if distributed uniformly throughout the tissues to elicit the current plasma concentration. | |||
|align="center"|L (Vd) | |||
|align="center"|D / C | |||
|- | |||
!Elimination Constant (Rate) | |||
|The rate at which the drug is removed from the body. | |||
|align="center"|h-1 (Ke) | |||
|align="center"|ln(2) / t1/2 or CL / Vd | |||
|- | |||
!Bioavailability | |||
|How much of the administered dose is available for actual use by the body. | |||
|no units as expressing a fraction (f) | |||
|align="center"|100 × (AUC (po)×D (iv))/(AUC (iv)×D (po)) | |||
AUC = Area under curve po = oral administration iv = intravenous administration | |||
|- | |||
!Cmax or Cmin | |||
|The maximum (Cmax) / minimum (Cmin) plasma drug concentration reached following drug administration | |||
|align="center"|µg/ml (Cmax or Cmin) | |||
|Identified via direct measurement of plasma C | |||
|- | |||
!tmax | |||
|The time it takes for a drug to reach Cmax following administration | |||
|align="center"|h (tmax) | |||
|Identified via direct measurement of plasma C over time | |||
|- | |||
!Half-life | |||
|The time it takes for a drug to reach half its original concentration | |||
|align="center"|h (t1/2) | |||
|align="center"|ln(2) / Ke | |||
|- | |||
!Drug Clearance | |||
|The volume of plasma cleared of the drug over a set time | |||
|align="center"|l/h (CL) | |||
|align="center"|Vd x Ke or D / Area under curve | |||
|} | |||
==Common Drug-Drug Interactions== | |||
It is important to be aware of the interactions that can occur between concomitantly administered drugs, as they may effect efficacy and/or toxicity, or produce adverse side effects. Such interactions could for example affect drug absorption, drug bioavailability or efficacy, or combine to produce unwanted metabolites, as well as possibly having effects on clinical analyses. If a combination of two drugs decreases the effect of one or both of them, the interaction is termed an antagonistic effect; however if, conversely, a combination of two drugs enhances the effect of one or both of them, the interaction is termed a synergistic effect. Drugs that act on the cardiovascular system are high in interactivity, which is an issue as cardiovascular patients normally receive more than one drug. Some common drug—drug interactions related to cardiovascular drugs are listed below: | |||
{| class="wikitable" border="0" cellpadding="0" cellspacing="0" | |||
|- | |||
!Drug | |||
!Drugs that <big>↑</big> drug action | |||
!Drugs that <big>↓</big> drug action | |||
|- | |||
!Digoxin | |||
|valign="top"| | |||
*Diuretics | |||
*Antiarrhythmics | |||
*Macrolide antibiotics | |||
*Cholestyramine | |||
*Neomycin | |||
*Keto- and intraconazole | |||
*Calcium antagonists | |||
*Cyclosporine, indomethacin | |||
*HMG CoA reductase inhibitors | |||
*Benzodiazepines | |||
*Amiodarone | |||
*Verapamil | |||
|valign="top"| | |||
*Rifampicin | |||
*Antacids (liquid) | |||
|- | |||
!Warfarin | |||
|valign="top"| | |||
*Furosemide | |||
*Amiodarone | |||
*Sulfa | |||
*Macrolide and quinolone antibiotics | |||
*NSAIDs | |||
|valign="top"| | |||
*Azathioprine | |||
*Phenobarbitone | |||
*Carbamazepine | |||
*Dexamethasone | |||
*Prednisolone | |||
*Rifampicin | |||
*Vitamin K | |||
*Raloxifene | |||
|- | |||
!Clopidogrel | |||
|valign="top"| | |||
*Rifampicin | |||
*Caffeine | |||
*Methylxanthines | |||
*Phosphodiesterase inhibitors | |||
|valign="top"| | |||
*Statins | |||
*Calcium channel blockers | |||
*Warfarin | |||
*Proton pump inhibitors | |||
|- | |||
!Furosemide | |||
| | |||
|valign="top"| | |||
*NSAIDs | |||
*Phenytoin | |||
*Colesevelam | |||
|- | |||
!ACE Inhibitors | |||
|valign="top"| | |||
*NSAIDs | |||
*Probenecid | |||
*Calcium channel blockers | |||
|valign="top"| | |||
*Indomethacin | |||
*Antacids | |||
|- | |||
!ß-blockers | |||
|valign="top"| | |||
*Amiodarone | |||
*Calcium channel blockers | |||
*Diltiazem | |||
*Phenoxybenzamine | |||
|valign="top"| | |||
*Phenobarbital | |||
*Rifampicin | |||
*Cimetidine | |||
*Antacids (liquid) | |||
*NSAIDs | |||
|- | |||
!Statins | |||
|valign="top"| | |||
*Amiodarone | |||
*Verapamil | |||
*Fibrates | |||
*Amprenavir | |||
*Diltiazem | |||
|valign="top"| | |||
*Nevirapine | |||
*Rifampicin | |||
|} | |||
There are several mechanisms by which drugs are broken down by the body, usually via degradation by enzymes. One common family of enzymes involved in drug metabolismis the cytochrome P450 (CYP) family; a large, diverse group of enzymes that encourage oxidation of a variety of substrates, both endogenous (e.g. steroid hormones) and exogenous (e.g. toxins and drugs). CYP enzymes account for up to 75% of drug metabolism, aiding some drugs to form their active compounds but mostly deactivating drugs into inactive metabolites to be excreted. CYP enzymes can influence drug actions in several ways; they can increase drug metabolism (either increasing action via formation of the active by-product or decreasing action by metabolism of the active drug) or their action can be inhibited by drugs that compete for access to the CYP enzymes active site, preventing the normal interaction between drug and enzyme. Many drugs exert their interactions with other drugs viainterference with the CYP system. For example, if Drug A is metabolised by CYP and Drug B inhibits CYP activity, co-administration will result in a decreased bioavailability of Drug A. In humans there are 18 families and 43 subfamilies of the CYP group of enzymes, which target different substrates. Some CYP enzymes important in cardiovascular medicine, their cardiovascular-drug substrates and some of their interactions are shown in the table below: | |||
{| class="wikitable" border="0" cellpadding="0" cellspacing="0" | |||
|- | |||
!Enzyme | |||
!Substrates (e.g.) | |||
!Inhibitors (e.g.) | |||
!Inducers (e.g.) | |||
|- | |||
!CYP2C19 | |||
|valign="top"| | |||
*Clopidogrel | |||
*Propranolol | |||
*Warfarin | |||
|valign="top"| | |||
*Moclobemide | |||
*Chloramphenicol | |||
*Many anti-convulsants (Valproate) | |||
*Proton pump inhibitors (Omeprazole) | |||
|valign="top"| | |||
*Rifampicin | |||
*Carbamazepine | |||
*Prednisone | |||
|- | |||
!CYP3A4 | |||
|valign="top"| | |||
*Donepezil | |||
*Statins (Atorvastatin) | |||
*Ca-channel blockers (Nifedipine) | |||
*Amiodarone | |||
*Dronedarone | |||
*Quinidine | |||
*PDE5 Inhibitors (Sildenafil) | |||
*Kinins | |||
*Caffeine | |||
*Eplerenone | |||
*Propranolol | |||
*Salmeterol | |||
*Warfarin | |||
*Clopidogrel | |||
|valign="top"| | |||
*Protease inhibitors (Ritonavir) | |||
*Macrolides (Clarithromycin) | |||
*Chloramphenicol | |||
*Nefazodone | |||
*Some Ca-channel blockers (Verapamil) | |||
*Cimetidine | |||
*Some azole anti-fungals (Ketaconazole) | |||
*Grapefruit juice | |||
|valign="top"| | |||
*Some anti-convulsants (Carbamazepine) | |||
*Baribiturates (Phenobarbital) | |||
*St. John’s Wort | |||
*Some reverse transcriptase inhibitors (Efavirenz) | |||
*Some Hypoglycaemics (Pioglitazone) | |||
*Glucocorticoids | |||
*Modafinil | |||
|- | |||
!CYP2C9 | |||
|valign="top"| | |||
*Fluvastatin | |||
*Angiotensin receptor II agonists (losartan) | |||
*Warfarin | |||
*Torasemide | |||
|valign="top"| | |||
*Some azole anti-fungals (Fluconazole) | |||
*Amiodarone | |||
*Antihistamines (Cyclizine) | |||
*Chloramphenicol | |||
*Fluvastatin | |||
*Fluvoxamine | |||
*Probenecid | |||
*Sertraline | |||
|valign="top"| | |||
*Rifampicin | |||
*Secobarbital | |||
|- | |||
!CYP2D6 | |||
|valign="top"| | |||
*ß-blockers (Propranolol) | |||
*Class I anti-arrythmics (Flecainide) | |||
*Donepezil | |||
|valign="top"| | |||
*SSRIs (Fluoxetine) | |||
*Quinidine | |||
*Sertraline | |||
*Terbinafine | |||
*Amiodarone | |||
*Cinacalcet | |||
*Ritonavir | |||
*Antipsychotics (Haloperidol) | |||
*Antihistamines (Promethazine) | |||
*Metoclopramide | |||
*Ranitidine | |||
*Mibefradil | |||
|valign="top"| | |||
*Rifampicin | |||
*Dexamethasone | |||
*Glutethimide | |||
|} | |||
In addition to drug-drug interactions, the actions of many drugs are also affected by food or drink. For example, care should be taken with alcohol consumption with many kinds of drugs, as it can put stress on the liver which is already working hard to metabolise drugs in the body. Grapefruit juice too can cause issues, as it is known to inhibit CYP3a. For more information of interactions between drugs and food/drinks see this guide: [http://www.fda.gov/downloads/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/GeneralUseofMedicine/UCM229033.pdf General Use of Medicine] | |||
==Common Cardiovascular Drugs== | |||
{| class="wikitable" border="0" cellpadding="0" cellspacing="0" | |||
|- | |||
|+Cardiovascular Drugs | |||
|- | |||
!Drug Type | |||
!Examples (generic name) | |||
!Indications | |||
!Typical Dosage | |||
!Guidelines / Class of Indication | |||
!Side Effects (Prevalence %) | |||
|- | |||
|colspan="6" bgcolor="#E6E6FA"|'''Anti-hypertensives''' | |||
|- | |||
|rowspan="2"|Diuretics | |||
|rowspan="2"|Furosemide | |||
|Oedema | |||
|Furosemide: 20-40mg once daily | |||
| | |||
|rowspan="2" valign="top"|Mild gastro-intestinal disturbances, pancreatitis, hepatic encephalopathy, postural hypotension, temporary increase in serum-cholesterol and triglyceride concentration, hyperglycaemia, acute urinary retention, electrolyte disturbances, metabolic alkalosis, blood disorders, hyperuricaemia, visual disturbances, tinnitus and deafness, and hypersensitivity reactions (including rash, photosensitivity, and pruritus). | |||
|- | |||
|Resistant Hypertension | |||
|Furosemide: 40-80mg once daily | |||
|Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IC <cite>Esc1</cite> | |||
|- | |||
|rowspan="4"|ACE Inhibitors | |||
|rowspan="4"|Lisinopril, Captopril | |||
|Hypertension | |||
|Lisinopril: 10mg once daily initially, maintenance 20mg once daily | |||
|Hypertension: Class IA <cite>Esc2</cite> | |||
Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IA <cite>Esc1</cite> | |||
Hypertension in diabetics: Class IA <cite>Esc3</cite> | |||
|rowspan="4" valign="top"|Hypotension (2.4%), renal impairment, persistent dry cough, angioedema, rash pancreatitis, upper respiratory-tract symptoms (2-10%), gastro-intestinal symptoms (1-2%), altered liver function tests, cholestatic jaundice, hepatitis, fulminant hepatic necrosis and failure, hyperkalaemia (2%), hypoglycaemia, blood disorders including thrombocytopenia, leucopenia, neutropenia, headache (3%), dizziness (2-12%), fatigue, malaise, taste disturbance, paraesthesia, bronchospasm, fever, serositis, vasculitis, myalgia (3%), arthralgia, positive antinuclear antibody, raised erythrocyte sedimentation rate, eosinophilia, leucocytosis, and photosensitivity. | |||
|- | |||
|Heart Failure | |||
|Lisinopril: 2.5-5mg once daily initially, maintenance 20-35mg once daily | |||
|Post STEMI: Class IA <cite>Esc4</cite> | |||
Diabetic patients: Class IC <cite>Esc2</cite> | |||
Symptomatic (NYHA class II-IV) HF: Class IA; Acute heart failure with ACS: Class IA <cite>Esc1</cite> | |||
|- | |||
|Prophylaxis Following MI | |||
|Lisinopril: 5mg within 24 hours of onset, 5mg after 24 hours, then 10mg after 48 hours | |||
| | |||
|- | |||
|Diabetic nephropathy | |||
|Lisinopril: 10-20mg once daily | |||
| | |||
|- | |||
|rowspan="3"|Angiotensin Receptor Blockers | |||
|rowspan="3"|Losartan, Candesartan | |||
|Hypertension | |||
|Losartan: 50mg once daily | |||
|Hypertension: Class IA <cite>Esc2</cite> | |||
Hypertension in diabetics: Class IA <cite>Esc3</cite> | |||
|rowspan="3" valign="top"|Gastro-intestinal disturbances (<3%), dizziness (14%), angina, palpitation, oedema, dyspnoea, headache (14%), malaise, urticaria, pruritus, rash; | |||
|- | |||
|Left ventricular hypertrophy | |||
|Losartan: 12.5-150mg daily | |||
|LVH: Class IB <cite>Esc4</cite> | |||
|- | |||
|Diabetic nephropathy | |||
|Losartan: 50mg daily | |||
| | |||
|- | |||
|rowspan="2"|Angiotensin Receptor Neprilysin Inhibitors | |||
|rowspan="2"|Sacubitril/Valsartan (Entresto) | |||
|Heart Failure with reduced ejection fraction (HFrEF) | |||
|49/51mg twice daily initially; target dose 97/103mg twice daily | |||
|Symptomatic (NYHA class II-IV) HF: Class IA <cite>Esc1</cite> | |||
|rowspan="2" valign="top"|Hypotension (18%), renal dysfunction (3%), hyperkalemia (16%), cough, dizziness, angioedema (<1%), fatigue. Should not be used with ACE inhibitors (36-hour washout period required) or in pregnancy. | |||
|- | |||
|Heart Failure with mildly reduced or preserved ejection fraction | |||
|49/51mg twice daily initially; target dose 97/103mg twice daily | |||
|HFmrEF and HFpEF: Class IA <cite>Esc1</cite> | |||
|- | |||
|rowspan="3"|Alpha Blockers | |||
|rowspan="3"|Doxazosin, Prazosin | |||
|Hypertension | |||
|Doxazosin: 1mg once daily initially, increased to 4-8mg once daily | |||
| | |||
|rowspan="3" valign="top"|Drowsiness, hypotension (notably postural hypotension) (10-70% initially), syncope (1%), asthenia, dizziness, depression, headache (8-18%), dry mouth, gastro-intestinal disturbances, oedema, blurred vision (<5%), intra-operative floppy iris syndrome, rhinitis (<4%), erectile disorders (including priapism), tachycardia and palpitations (7-14%), gastrointestinal side-symptoms (4-5%), hypersensitivity reactions including rash, pruritus and angioedema. | |||
|- | |||
|Congestive Heart Failure | |||
|Doxazosin: 4-16mg daily | |||
| | |||
|- | |||
|Raynaud's Syndrome | |||
|Doxazosin: 1-4mg daily | |||
| | |||
|- | |||
|rowspan="3"|Beta Blockers | |||
|rowspan="3"|Atenolol, Metoprolol, Propranolol | |||
|Hypertension | |||
|Atenolol: 25-50mg daily | |||
Metoprolol: 100-200mg daily | |||
| | |||
|rowspan="3" valign="top"|Gastro-intestinal disturbances (2-4%); bradycardia, heart failure, hypotension, conduction disorders, peripheral vasoconstriction, bronchospasm, dyspnoea; headache, fatigue, sleep disturbances (2-5%), paraesthesia, dizziness (2-5%), vertigo, psychoses; sexual dysfunction; purpura, thrombocytopenia; visual disturbances; exacerbation of psoriasis, alopecia; rarely rashes and dry eyes. | |||
|- | |||
|Angina | |||
|Atenolol: 100mg once/twice daily | |||
Metoprolol: 50-100mg twice daily | |||
|ACS: Class IIaB <cite>Esc2</cite> | |||
Atrial fibrillation: Class IA | Angina in symptomatic (NYHA class II-IV) HF and LVD: Class IA <cite>Esc1</cite> | ||
Polymorphic VT: Class IB | |- | ||
|Arrhythmias | |||
|Atenolol: 50-100mg daily | |||
Metoprolol: 100-200mg daily | |||
|Atrial fibrillation: Class IA; Polymorphic VT: Class IB <cite>Esc4</cite> | |||
Symptomatic (NYHA class II-IV) HF, LVD and AF: Class IA; Management of VA in HF: Class IA <cite>Esc1</cite> | |||
Symptomatic (NYHA class II-IV) HF, LVD and AF: Class IA | |||
Management of VA in HF: Class IA | |||
SVT: Class IIbC; Wide QRS-complex tachycardia of unknown origin: Class IIIC; Sinus tachycardia: Class IC; Poorly tolerated AVNRT with haemodynamic intolerance: Class IIaC; Recurrent symptomatic AVNRT: Class IC; Documented PSVT with only dual AV-nodal pathways or single echo beats demonstrated during electrophysiological study and no other identified cause of arrhythmia: Class IC; Infrequent, well tolerated AVNRT: Class IB; Focal junction tachycardia: Class IIaC; Nonparoxysmal junctional tachycardia: Class IIaC; WPW Syndrome: Class IIaC; AVRT, poorly tolerated: Class IIbC; Since or infrequent AVRT episode(s): Class IIaB; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IC; AF (Poorly tolerated): Class IIaC; AF (Stable flutter): Class IC; Prophylaxis of SVT during pregnancy: Class IIaB <cite>Acc5</cite> | |||
SVT: Class IIbC | |- | ||
Wide QRS-complex tachycardia of unknown origin: Class IIIC | |rowspan="3"|Calcium Channel Blockers | ||
Sinus tachycardia: Class IC | |rowspan="3"|Nifedipine, Verapamil, Diltiazem | ||
Poorly tolerated AVNRT with haemodynamic intolerance: Class IIaC | |Hypertension | ||
Recurrent symptomatic AVNRT: Class IC | |Nifedipine: 30-60mg once daily (slow-release) | ||
Documented PSVT with only dual AV-nodal pathways or single echo beats demonstrated during electrophysiological study and no other identified cause of arrhythmia: Class IC | |Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IA <cite>Esc1</cite> | ||
Infrequent, well tolerated AVNRT: Class IB | |rowspan="3" valign="top"|Gastro-intestinal disturbance (2-11%); hypotension (1-5%), oedema (7-29%), vasodilatation, palpitation; headache (7-35%), dizziness (3-27%), lethargy (4-6%), asthenia (10-12%); less commonly tachycardia (<1-7%), syncope (<1%), chills, nasal congestion, dyspnoea (<3%), anxiety, sleep disturbance (<2%), vertigo (<3%), migraine, paraesthesia, tremor (1-8%), polyuria, dysuria, nocturia, erectile dysfunction (<2%), epistaxis, myalgia, joint swelling, visual disturbance (<2%), sweating (<2%), hypersensitivity reactions (<1%); rarely anorexia, gum hyperplasia, mood disturbances, hyperglycaemia, male infertility, purpura (<1%), and photosensitivity reactions (<1%); also reported dysphagia, intestinal obstruction, intestinal ulcer, bezoar formation, gynaecomastia, agranulocytosis, and anaphylaxis; | ||
Focal junction tachycardia: Class IIaC | |- | ||
Nonparoxysmal junctional tachycardia: Class IIaC | |Raynaud's Syndrome | ||
WPW Syndrome: Class IIaC | |Nifedipine: 20-40mg once daily (slow-release) | ||
AVRT, poorly tolerated: Class IIbC | | | ||
Since or infrequent AVRT episode(s): Class IIaB | |- | ||
Acute treatment of Focal Atrial Tachycardia: Class IIaC | |Angina (prophylaxis) | ||
Prophylactic therapy for AT: Class IC | |Nifedipine: 30-60mg once daily (slow-release) | ||
AF (Poorly tolerated): Class IIaC | |Angina in symptomatic (NYHA class II-IV) HF and LVD: Class IIaA <cite>Esc1</cite> | ||
AF (Stable flutter): Class IC | |- | ||
Prophylaxis of SVT during pregnancy: Class IIaB | |colspan="6" bgcolor="#E6E6FA"|'''Heart Failure Medications''' | ||
|- | |||
|rowspan="2"|Angiotensin Receptor Neprilysin Inhibitors | |||
|rowspan="2"|Sacubitril/Valsartan (Entresto) | |||
Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IA | |Heart Failure with reduced ejection fraction (HFrEF) | ||
|49/51mg twice daily initially; target dose 97/103mg twice daily | |||
|Symptomatic (NYHA class II-IV) HF: Class IA <cite>Esc1</cite> | |||
|rowspan="2" valign="top"|Hypotension (18%), renal dysfunction (3%), hyperkalemia (16%), cough, dizziness, angioedema (<1%), fatigue. Should not be used with ACE inhibitors (36-hour washout period required) or in pregnancy. | |||
|- | |||
Anti-Arrhythmics Class I (sodium channel blockers) Flecainide, Lidocaine, Procainamide Ventricular Arrhythmias Flecainide: 50-100mg twice daily | |Heart Failure with mildly reduced or preserved ejection fraction | ||
|49/51mg twice daily initially; target dose 97/103mg twice daily | |||
Sustained VT and VF: Class IIbC | |HFmrEF and HFpEF: Class IA <cite>Esc1</cite> | ||
|- | |||
|rowspan="3"|SGLT2 Inhibitors | |||
|rowspan="3"|Empagliflozin, Dapagliflozin, Canagliflozin, Sotagliflozin | |||
|Heart Failure with reduced ejection fraction | |||
|Empagliflozin: 10mg once daily | |||
Dapagliflozin: 10mg once daily | |||
|Symptomatic (NYHA class II-IV) HF with reduced EF: Class IA <cite>Esc1</cite> | |||
|rowspan="3" valign="top"|Genital mycotic infections (10%), urinary tract infections (4-8%), volume depletion (1-3%), hypotension, euglycemic diabetic ketoacidosis (<0.1%), acute kidney injury (2%), hypoglycemia (when used with insulin or insulin secretagogues), increased urination, thirst, and cholesterol levels. Rare serious adverse effects include Fournier's gangrene and lower limb amputations (particularly with canagliflozin). | |||
|- | |||
|Heart Failure with preserved ejection fraction | |||
|Empagliflozin: 10mg once daily | |||
Dapagliflozin: 10mg once daily | |||
|HFmrEF and HFpEF: Class I <cite>Esc1</cite> | |||
|- | |||
|Type 2 Diabetes with high CV risk or CKD | |||
|Empagliflozin: 10-25mg once daily | |||
Dapagliflozin: 10mg once daily | |||
Canagliflozin: 100-300mg once daily | |||
|Type 2 diabetes with CV disease: Class IA <cite>Esc2</cite> | |||
Type 2 diabetes with CKD: Class I <cite>Esc1</cite> | |||
|- | |||
|colspan="6" bgcolor="#E6E6FA"|'''Anti-Arrhythmics''' | |||
|- | |||
|Class I (sodium channel blockers) | |||
|Flecainide, Lidocaine, Procainamide | |||
|Ventricular Arrhythmias | |||
|Flecainide: 50-100mg twice daily | |||
|Sustained VT and VF: Class IIbC <cite>Esc4</cite> | |||
Pre-excited SVT/AF: Class IB; Wide QRS-complex tachycardia of unknown origin: Lidocaine (Class IIbB) / Procainamide (Class IB); Wide QRS-complex tachycardia of unknown origin with LVD: Class IB; Focal junction tachycardia: Class IIaC; WPW Syndrome: IIaC; AVRT, poorly tolerated: IIaC; Single or infrequent AVRT episode(s): Class IIbC; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IIaC; AF (Stable flutter): Class IIbA; Prophylaxis of SST during pregnancy: Class IIbB <cite>Acc5</cite> | |||
Pre-excited SVT/AF: Class IB | |Oedema, pro-arrhythmic effects (1-13%); dyspnoea; nervous-system side-effects including dizziness, asthenia, fatigue, fever; visual disturbances (13-28%); rarely pneumonitis, hallucinations, depression, confusion, amnesia, dyskinesia, convulsions, peripheral neuropathy; also reported gastro-intestinal disturbances (1-4%), anorexia, hepatic dysfunction, flushing, syncope, drowsiness, tremor, vertigo, headache, anxiety, insomnia, ataxia, paraesthesia, anaemia, leucopenia, thrombocytopenia, corneal deposits, tinnitus, increased antinuclear antibodies, hypersensitivity reactions (including rash, urticaria, and photosensitivity), increased sweating. | ||
Wide QRS-complex tachycardia of unknown origin: Lidocaine (Class IIbB) / Procainamide (Class IB) | |- | ||
Wide QRS-complex tachycardia of unknown origin with LVD: Class IB | |Class II (Beta blockers) | ||
Focal junction tachycardia: Class IIaC | |(See above) | ||
WPW Syndrome: IIaC | |(See above) | ||
AVRT, poorly tolerated: IIaC | |(See above) | ||
Single or infrequent AVRT episode(s): Class IIbC | | | ||
Acute treatment of Focal Atrial Tachycardia: Class IIaC | |(See above) | ||
Prophylactic therapy for AT: Class IIaC | |- | ||
AF (Stable flutter): Class IIbA | |Class III (Potassium channel blockers) | ||
Prophylaxis of | |Amiodarone, Sotalol | ||
|Ventricular, Arrhythmias | |||
|Amiodarone: 200mg 2-3 times daily | |||
|Sustained VT and VF: Class IIaC; Polymorphic VT: Class IC <cite>Esc4</cite> | |||
Sustained VT and VF: Class IIaC | |||
Polymorphic VT: Class IC | |||
Management of VA in HF: Class IA; Prevention of VA in HF: Class IIbB <cite>Esc1</cite> | |||
Management of VA in HF: Class IA | |||
Prevention of VA in HF: Class IIbB | |||
SVT: Class IIBC; Wide QRS-complex tachycardia of unknown origin: Class IB; Wide QRS-complex tachycardia of unknown origin with LVD: Class IB; Recurrent AVNRT unresponsive to beta blocker or calcium-channel blocker and patient not desiring RF ablation: Class IIbC; Focal junction tachycardia: Class IIaC; WPW Syndrome: IIaC; AVRT, poorly tolerated: Class IIaC; Since or infrequent AVRT episode(s): Class IIbB; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IIaC; AF (Poorly tolerated): Class IIbC; AF (Stable flutter): Class IIbC; Prophylaxis of SVT during pregnancy: Class IIIC <cite>Acc5</cite> | |||
SVT: Class IIBC | |Gastro-intestinal disturbances (2-20%)), taste disturbances, hepatic disturbances (up to 50%); bradycardia; pulmonary toxicity (1-17%); tremor (9-59%), sleep disorders; hypothyroidism (5-10%), hyperthyroidism (5-10%); reversible corneal microdeposits (up to 98%); phototoxicity, persistent slate-grey skin discoloration (1-7%), injection-site reactions; less commonly onset or worsening of arrhythmia, conduction disturbances, peripheral neuropathy (1-105) and myopathy; very rarely sinus arrest, bronchospasm, ataxia (2-37%), benign intracranial hypertension, headache, vertigo, epididymo-orchitis, impotence, haemolytic or aplastic anaemia, thrombocytopenia, rash, hypersensitivity including photosensitivity (2-20%), anaphylaxis on rapid injection, hypotension (10-30%), respiratory distress syndrome, sweating, and hot flushes | ||
Wide QRS-complex tachycardia of unknown origin: Class IB | |- | ||
Wide QRS-complex tachycardia of unknown origin with LVD: Class IB | |Class IV (Calcium channel blockers) | ||
Recurrent AVNRT unresponsive to beta blocker or calcium-channel blocker and patient not desiring RF ablation: Class IIbC | |(See above) | ||
Focal junction tachycardia: Class IIaC | |(See above) | ||
WPW Syndrome: IIaC | |(See above) | ||
AVRT, poorly tolerated: Class IIaC | | | ||
Since or infrequent AVRT episode(s): Class IIbB | |(See above) | ||
Acute treatment of Focal Atrial Tachycardia: Class IIaC | |- | ||
Prophylactic therapy for AT: Class IIaC | |rowspan="2"| | ||
AF (Poorly tolerated): Class IIbC | |rowspan="2"|Digoxin | ||
AF (Stable flutter): Class IIbC | |Supra-ventricular Arrhythmias | ||
Prophylaxis of SVT during pregnancy: Class IIIC Gastro-intestinal disturbances (2-20%)), taste disturbances, hepatic disturbances (up to 50%); bradycardia; pulmonary toxicity (1-17%); tremor (9-59%), sleep disorders; hypothyroidism (5-10%), hyperthyroidism (5-10%); reversible corneal microdeposits (up to 98%); phototoxicity, persistent slate-grey skin discoloration (1-7%), injection-site reactions; less commonly onset or worsening of arrhythmia, conduction disturbances, peripheral neuropathy (1-105) and myopathy; very rarely sinus arrest, bronchospasm, ataxia (2-37%), benign intracranial hypertension, headache, vertigo, epididymo-orchitis, impotence, haemolytic or aplastic anaemia, thrombocytopenia, rash, hypersensitivity including photosensitivity (2-20%), anaphylaxis on rapid injection, hypotension (10-30%), respiratory distress syndrome, sweating, and hot flushes | |Acute: 0.75-1.5mg over 24 hours; Maintenance: 125-150µg daily | ||
|SVT: Class IIbC; WPW Syndrome: Class IIIC; AVRT, poorly tolerated: Class IIIC; Since or infrequent AVRT episode(s): Class IIIC; Prophylaxis of SVT during pregnancy: Class IC <cite>Acc5</cite> | |||
|rowspan="2" valign="top"|Gastro-intestinal disturbances (vomiting, diarrhoea, anorexia, abdominal pain) (25%); arrhythmias (up to 50%), AV conduction disturbances (50%); nervous system disturbances (dizziness, apathy, confusion, headache, fatigue, weakness) (25%); blurred or yellow vision; rash, eosinophilia, depression, anorexia, intestinal ischaemia and necrosis, psychosis, gynaecomastia on long-term use, and thrombocytopenia. | |||
Maintenance: 125-150µg daily | |- | ||
SVT: Class IIbC | |Heart Failure | ||
WPW Syndrome: Class IIIC | |62.5-125 µg daily | ||
AVRT, poorly tolerated: Class IIIC | |Symptomatic (NYHA class II-IV) HF: Class IIbB | ||
Since or infrequent AVRT episode(s): Class IIIC | |||
Prophylaxis of SVT during pregnancy: Class IC Gastro-intestinal disturbances (vomiting, diarrhoea, anorexia, abdominal pain) (25%); arrhythmias (up to 50%), AV conduction disturbances (50%); nervous system disturbances (dizziness, apathy, confusion, headache, fatigue, weakness) (25%); blurred or yellow vision; rash, eosinophilia, depression, anorexia, intestinal ischaemia and necrosis, psychosis, gynaecomastia on long-term use, and thrombocytopenia | |||
Symptomatic (NYHA class II-IV) HF: Class IIbB | |||
Symptomatic (NYHA class II-IV) HF, LVD and AF: Class IB; Acute HF with AF and VT: Class IC <cite>Esc1</cite> | |||
|- | |||
|colspan="6" bgcolor="#E6E6FA"|'''Anti-platelet Drugs''' | |||
|- | |||
|rowspan="7"| | |||
|rowspan="2"|Aspirin | |||
|Prevention of thrombotic cerebro- or cardio-vascular disease | |||
|75mg once/day | |||
|Prevention in AF: Class IC; Prevention in diabetic patients: IIaB <cite>Esc2</cite> | |||
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA <cite>Esc1</cite> | |||
Prevention in hypertensive patients with CV events: Class IA; Prevention in hypertensive patients without CV history but with reduced renal function/high risk: Class IIbA <cite>Esc3</cite> | |||
Prevention in | |||
Post-MI: Class Ia <cite>Esc3</cite> | |||
|rowspan="2" valign="top"|Bronchospasm (10-30% in asthmatics); gastro-intestinal irritation (up to 83%), gastro-intestinal haemorrhage (occasionally major), also other haemorrhage (e.g. intracranial (0.5%), subconjunctival), chest pain (8.3%), oedema (4.5%), hypertension (4.3%). | |||
Prevention in | |- | ||
|Pain / pyrexia | |||
|300-600mg every 4-6 hours as necessary | |||
| | |||
|- | |||
|rowspan="3"|Clopidogrel | |||
|Prevention of thrombotic events (esp. when warfarin not tolerated) | |||
|75mg once/day | |||
|Prevention in diabetic patients: IIaB; Primary and secondary prevention of stroke: Class IB <cite>Esc2</cite> | |||
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA | Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA <cite>Esc1</cite> | ||
Acute phase of coronary artery syndrome: Class IB; Non-cardioembolic cerebral ischaemic events: Class IA <cite>Esc3</cite> | |||
|rowspan="3" valign="top"|Dyspepsia (5.2%), abdominal pain (5.6%), diarrhoea (4.5%); bleeding disorders including gastro-intestinal (2.0%) and intracranial (0.4%), nausea (3.4%), vomiting, gastritis, flatulence, constipation, gastric and duodenal ulcers, headache (7.6%), epistaxis (2.9%), dizziness (6.2%), paraesthesia, leucopenia, decreased platelets (very rarely severe thrombocytopenia), eosinophilia, rash (4.2%), pruritus (3.3%), vertigo, colitis, pancreatitis, hepatitis (<1%), acute liver failure, hypertension (4.3%), chest pain (8.3%), oedema (4.1%), vasculitis, confusion, hallucinations, taste disturbance, cough (3.9%), fatigue (4.8%) stomatitis, bronchospasm, interstitial pneumonitis, pyrexia (2.2%), blood disorders including thrombocytopenic purpura (5.3%), agranulocytosis, neutropenia (0.04%) and pancytopenia and hypersensitivity-like reactions (<0.1%)including fever, glomerulonephritis, arthralgia, Stevens-Johnson syndrome, toxic epidermal necrolysis, lichen planus. | |||
Acute phase of coronary artery syndrome: Class IB | |- | ||
Non-cardioembolic cerebral ischaemic events: Class IA Dyspepsia (5.2%), abdominal pain (5.6%), diarrhoea (4.5%); bleeding disorders including gastro-intestinal (2.0%) and intracranial (0.4%), nausea (3.4%), vomiting, gastritis, flatulence, constipation, gastric and duodenal ulcers, headache (7.6%), epistaxis (2.9%), dizziness (6.2%), paraesthesia, leucopenia, decreased platelets (very rarely severe thrombocytopenia), eosinophilia, rash (4.2%), pruritus (3.3%), vertigo, colitis, pancreatitis, hepatitis (<1%), acute liver failure, hypertension (4.3%), chest pain (8.3%), oedema (4.1%), vasculitis, confusion, hallucinations, taste disturbance, cough (3.9%), fatigue (4.8%) stomatitis, bronchospasm, interstitial pneumonitis, pyrexia (2.2%), blood disorders including thrombocytopenic purpura (5.3%), agranulocytosis, neutropenia (0.04%) and pancytopenia and hypersensitivity-like reactions (<0.1%)including fever, glomerulonephritis, arthralgia, Stevens-Johnson syndrome, toxic epidermal necrolysis, lichen planus | |Acute myocardial infarction | ||
|300mg daily initially then 75mg once/day | |||
|Post STEMI: Class IA <cite>Esc4</cite> | |||
Post STEMI: Class IA | |- | ||
|Acute coronary syndrome | |||
ACS: Class IIaC | |300mg daily initially then 75mg once/day | ||
|ACS: Class IIaC <cite>Esc2</cite> | |||
|- | |||
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA | |Prasugrel | ||
|Prevention of thrombotic events. | |||
|60mg bolus then 5-10mg once daily | |||
|Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA <cite>Esc1</cite> | |||
Acute phase of coronary artery syndrome: Class IB <cite>Esc3</cite> | |||
|Haemorrhage (11.3%) (including gastro-intestinal (1.5%) and intracranial), haematoma, haematuria, hypertension (7.5%), hypotension (3.9%), headache (5.5%), back pain (5.0%), dyspnoea (4.9%), nausea (4.6%), dizziness (4.1%), cough (3.9%), fatigue (3.7%), chest pain (3.1%), arrhythmias including atrial fibrillation (2.9%) and bradycardia (2.9%), rash (2.8%), pyrexia (2.7%), oedema (2.7%), diarrhoea (2.3%), hypercholesterolaemia/hyperlipidaemia (7.5%), anaemia, rash,hypersensitivity reactions including angioedema (0.06%), thrombocytopenia (0.06%), thrombotic thrombocytopenic purpura. | |||
Acute phase of coronary artery syndrome: Class IB | |- | ||
|Ticragelor | |||
|Prevention of thrombotic events. | |||
|180mg bolus then 90mg twice daily | |||
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA | |Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA <cite>Esc1</cite> | ||
Acute phase of coronary artery syndrome: Class IB <cite>Esc3</cite> | |||
|Dyspnoea (13.8%), haemorrhage, bruising; nausea (4.3%), vomiting, diarrhoea (3.7%), hypertension (3.8%), hypotension (3.2%), back pain (3.6%), abdominal pain, dyspepsia, gastritis, dizziness (4.5%), chest pain (3.7%), headache (6.5%), cough (4.9%), rash, pruritus, fatigue (3.2%), constipation, arrhythmias including atrial fibrillation (4.2%), paraesthesia, confusion, hyperuricaemia, raised serum creatinine (7.4%), vertigo. | |||
|- | |||
|colspan="6" bgcolor="#E6E6FA"|'''Vitamin K Antagonists''' | |||
|- | |||
| | |||
|Warfarin | |||
|Prevention of thrombotic/ embolic events (esp. after prosthetic valve insertion) | |||
|5-10mg initially then tailored to individual (usually 3-9mg once daily at the same time) | |||
| | |||
|valign="top"|Haemorrhage, nausea, vomiting, diarrhoea, jaundice, hepatic dysfunction, pancreatitis, pyrexia, alopecia, purpura, rash, 'purple toes', skin necrosis (increased risk in patients with protein C or protein S deficiency) | |||
|- | |||
| | |||
|Acenocoumarol | |||
|Prevention of thrombotic/ embolic events (esp. after prosthetic valve insertion) | |||
|4mg initially, followed by 1-8mg daily | |||
| | |||
|valign="top"|Haemorrhage, nausea, vomiting, diarrhoea, jaundice, hepatic dysfunction, pancreatitis, pyrexia, alopecia, purpura, rash, 'purple toes', skin necrosis (increased risk in patients with protein C or protein S deficiency) | |||
|- | |||
|colspan="6" bgcolor="#E6E6FA"|'''Non-vitamin K Antagonist Oral Anticoagulants (NOACs)''' | |||
|- | |||
|rowspan="3"|Direct Thrombin Inhibitors | |||
|rowspan="3"|Dabigatran | |||
|Prevention of stroke and systemic embolism in non-valvular atrial fibrillation | |||
|150mg twice daily (110mg twice daily if >80 years, GFR 30-50 mL/min, or increased bleeding risk) | |||
|Prevention of stroke in AF: Class IA <cite>Esc3</cite> | |||
|rowspan="3" valign="top"|Dyspepsia (10%), gastritis, esophagitis, gastroesophageal reflux (1-3%), abdominal pain (3%), diarrhea (3%), bleeding (including gastrointestinal 1-2%, intracranial <1%), hypersensitivity reactions (<0.1%), hepatic dysfunction. Highest renal elimination (80%) among NOACs; contraindicated if CrCl <30 mL/min. | |||
|- | |||
|Treatment and prevention of deep vein thrombosis and pulmonary embolism | |||
|150mg twice daily after 5-10 days of parenteral anticoagulation | |||
|VTE treatment: Class IA <cite>Esc3</cite> | |||
|- | |||
|Prevention of VTE after knee or hip replacement | |||
|110mg 1-4 hours after surgery then 220mg once daily for 10 days (knee) or 28-35 days (hip) | |||
|VTE prophylaxis post-surgery: Class IA <cite>Esc3</cite> | |||
|- | |||
|rowspan="4"|Factor Xa Inhibitors | |||
|rowspan="4 de>|Rivaroxaban, Apixaban, Edoxaban | |||
|Prevention of stroke and systemic embolism in non-valvular atrial fibrillation | |||
|Rivaroxaban: 20mg once daily with food (15mg if CrCl 15-49 mL/min) | |||
Apixaban: 5mg twice daily (2.5mg twice daily if ≥2 of: age ≥80 years, weight ≤60kg, or serum creatinine ≥133μmol/L) | |||
Edoxaban: 60mg once daily (30mg if CrCl 15-50 mL/min or body weight ≤60kg) | |||
|Prevention of stroke in AF: Class IA <cite>Esc3</cite> | |||
|rowspan="4" valign="top"|Bleeding (major 2-3%, clinically relevant non-major 4-7%), nausea (1-3%), anemia (1-2%), rash (1-2%), abnormal liver function tests (1-2%), thrombocytopenia (<1%). | |||
Rivaroxaban and edoxaban must be taken with food. | |||
Varying degrees of renal elimination: edoxaban (50%), rivaroxaban (35%), apixaban (27%). | |||
Apixaban has lowest bleeding risk among NOACs. All contraindicated with CrCl <15 mL/min. | |||
|- | |||
|Treatment of deep vein thrombosis and pulmonary embolism | |||
|Rivaroxaban: 15mg twice daily for 21 days, then 20mg once daily | |||
Apixaban: 10mg twice daily for 7 days, then 5mg twice daily | |||
Edoxaban: 60mg once daily after 5-10 days of parenteral anticoagulation | |||
|VTE treatment: Class IA <cite>Esc3</cite> | |||
|- | |||
|Prevention of recurrent deep vein thrombosis and pulmonary embolism | |||
|Rivaroxaban: 10-20mg once daily | |||
Apixaban: 2.5mg twice daily | |||
Edoxaban: 60mg once daily (30mg if CrCl 15-50 mL/min or body weight ≤60kg) | |||
|VTE recurrence prevention: Class IA <cite>Esc3</cite> | |||
|- | |||
|Prevention of VTE after knee or hip replacement | |||
|Rivaroxaban: 10mg once daily for 2 weeks (knee) or 5 weeks (hip) | |||
Apixaban: 2.5mg twice daily for 10-14 days (knee) or 32-38 days (hip) | |||
|VTE prophylaxis post-surgery: Class IA <cite>Esc3</cite> | |||
|- | |||
|colspan="6" bgcolor="#E6E6FA"|'''Lipid-Lowering Drugs''' | |||
|- | |||
|rowspan="3"|Statins | |||
|rowspan="3"|Simvastatin, Atorvastatin | |||
|Primary hyper-cholesterolaemia, combined hyperlipidaemia | |||
|Simvastatin: 10-20mg once daily | |||
|Dyslipidaemia: Class IA; Low HDL-C: Class IIbB; Elderly patients with CVD: IB; Elderly patients with no CVD but CV risk factors: IIbB; Type I diabetes: IC; Patients with CKD: IIaC; Transplant patients: Class IIaB; PAD: Class IA; HIV patients: IIaC <cite>Esc2</cite> | |||
Hypertension in diabetics: Class IA; ACS: Class IA <cite>Esc3</cite> | |||
|rowspan="3" valign="top"|Oedema (2.7%), abdominal pain (5.9%), nausea (5.4%), atrial fibrillation (5.7%), constipation (2.2%), gastritis (4.9%), diabetes mellitus (4.2%), myalgia (3.7%), headache (2.5%), insomnia (4.0%), vertigo (4.5%), bronchitis (6.6%), sinusitis (2.3%), eczema (4.5%), urinary tract infection (3.2%) | |||
|- | |||
|Homozygous Familial Hypercholesterolemia | |||
|Simvastatin: 40mg once daily | |||
|Homozygous Familial Hypercholesterolemia: Class I <cite>Esc2</cite> | |||
|- | |||
|Secondary prevention in very high-risk patients | |||
|Atorvastatin: 40-80mg once daily | |||
|Very high-risk patients failing to achieve LDL-C goals on maximum tolerated statin: Class I <cite>Esc3</cite> | |||
|- | |||
|rowspan="3"|PCSK9 Inhibitors | |||
|rowspan="3"|Evolocumab, Alirocumab, Inclisiran | |||
|Primary hypercholesterolemia, Mixed Dyslipidemia | |||
|Evolocumab: 140mg every 2 weeks or 420mg once monthly | |||
Alirocumab: 75mg to 150mg every 2 weeks | |||
Inclisiran: 300mg initial dose, then 300mg at 3 months, followed by 300mg every 6 months | |||
|Primary hypercholesterolemia and Mixed Dyslipidemia: Class I <cite>Esc2</cite> | |||
|rowspan="3" valign="top"|Injection site reactions (5.9%), nasopharyngitis (5.5%), upper respiratory tract infections (2.3%), flu (3.1%), back pain (3.2%), hypersensitivity reactions including rash, pruritus (1.7%), and rare cases of neurocognitive effects such as memory loss or confusion. Inclisiran has less frequent administration (twice yearly) as a small interfering RNA that inhibits PCSK9 production in the liver. | |||
|- | |||
|Homozygous Familial Hypercholesterolemia | |||
|Evolocumab: 420mg once monthly (may increase to 420mg every 2 weeks if needed) | |||
|Homozygous Familial Hypercholesterolemia: Class I <cite>Esc2</cite> | |||
|- | |||
|Secondary prevention in very high-risk patients | |||
|Evolocumab: 140mg every 2 weeks | |||
Alirocumab: 75mg to 150mg every 2 weeks | |||
Inclisiran: 300mg initial dose, then 300mg at 3 months, followed by 300mg every 6 months | |||
|Very high-risk patients failing to achieve LDL-C goals on maximum tolerated statin: Class I <cite>Esc3</cite> | |||
|} | |||
==References== | |||
<biblio> | |||
#Esc1 pmid=22611136 | |||
#Esc2 pmid=17220161 | |||
#Esc3 pmid=22555213 | |||
#Esc4 pmid=22922416 | |||
#Acc5 pmid=14557344 | |||
#Esc6 pmid=21712404 | |||
</biblio> | |||
Latest revision as of 19:32, 13 May 2025
Heather Melrose, Jonas de Jong
Cardiovascular disease including heart disease, arrhythmias and hypertension, is the leading cause of morbidity and mortality in the Western world. There are numerous devastating conditions affecting the heart and/or the vasculature, leading to high demand for cardiovascular drugs. This chapter focuses on some key therapeutic targets within the cardiovascular system and the drugs used to combat cardiovascular disease.
Renin-Angiotensin-Aldosterone System
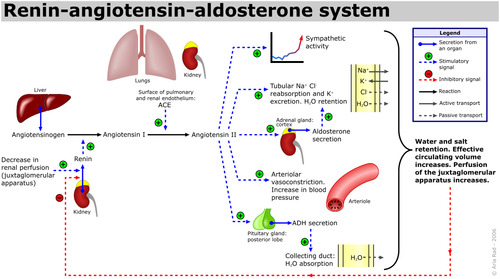
The renin-angiotensin-aldosterone system (RAAS) is an important hormone-based pathway within the body that regulates fluid balance and thus systemic blood pressure. The system is activated by decreases in blood volume or pressure detected in two ways: a drop in blood pressure detected by baroreceptors (pressure sensors) located in the carotid sinus or a drop in flow rate through the kidneys, detected by the juxtaglomerular apparatus. The body responds to these stimuli to effect a restoration in blood pressure via the actions of three hormones; renin, angiotensin and aldosterone. Following the detected drop in blood pressure, the enzyme renin is released from specialised cells within the kidney. The substrate of renin is the inactive precursor of angiotensin I, angiotensinogen. Angiotensin I is then enzymatically converted by angiotensin converting enzyme (ACE) into angiotensin II, a hormone with various actions throughout the body that ultimately increase blood pressure, restoring fluid balance within the body.
Angiotensin II causes increases in blood pressure by actions at various sites:
- Adrenal Glands: Angiotensin II augments release of the steroid hormone aldosterone, which acts locally to augment sodium retention and potassium secretion from the kidney. The net effect of this is water retention, thus restoring fluid balance.
- Kidneys: Angiotensin II also increases sodium retention via direct actions on renal proximal tubules, as well as affecting glomerular filtration rate and renal blood flow.
- Cardiovascular System: Angiotensin II is a potent endogenous vasoconstrictor, causing resistance arteries and veins to constrict, raising blood pressure. Furthermore in both the blood vessels and the heart, prolonged increases in Angiotensin II encourage cell growth and resultant hypertrophy.
- Central Nervous System: In the brain, Angiotensin II acts on the posterior pituitary gland, stimulating release of antidiuretic hormone (ADH, also known as Arginine Vasopressin (AVP)). ADH increases water reabsorption in the renal collecting ducts. Angiotensin II also acts on the subfornical organ within the brain to cause increased thirst, encouraging water intake.
Chronic activation of the RAAS system can lead to deleterious remodelling and increased inflammation in the heart, vasculature and kidneys, as well as hypertension and chronic kidney disease.
Neural Control of the Cardiovascular System
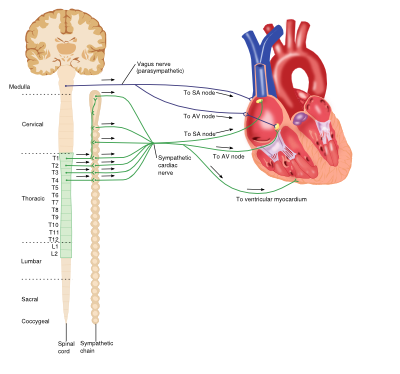
Sympathetic (Adrenergic) Nervous System
The adrenergic nervous system is a vital component of many processes throughout the body, including the cardiovascular system. Circulating catecholamines (e.g. adrenaline and noradrenaline) bind to and activate adrenergic receptors on cell membranes. Adrenergic receptors are a class of G-protein coupled receptors that elicit a variety of tissue-specific effects and exist in several subtypes.
Vasculature
The predominant receptor subtype present in blood vessels is the a1-adrenergic receptor, activation of which by catecholamine binding causes activation of the phospholipase-C (PLC), inositol triphosphate (IP3), diacylglycerol (DAG) intracellular signalling pathway. This ultimately results in myocyte contraction, vasoconstriction and consequent increases in systemic blood pressure.
Heart
Although the heart is myogenic, that is the impetus for contraction is self-initiated, the output of the heart is influenced by the central nervous system. The net effect of the sympathetic system on the heart is to increase cardiac output. The adrenergic receptors found in the heart belong to the ß-receptor subfamily and include ß1 and ß3 receptors. Catecholamine binding to ß1-receptors in the heart causes increases in cardiac output via a number of mechanisms: positive chronotropic effects, positive inotropic effects increased automaticity and conduction in both ventricular myocytes and the atrioventricular (AV) node. However ß3-receptor activation antagonises these actions, producing a negative inotropic effect and providing an inbuilt control system within the heart.
Prolonged increase catecholamine levels in the circulation (e.g. when secreted from adrenal tumours or times of stress) can lead to chronic cardiovascular problems such as hypertension and arrhythmias.
Parasympathetic Nervous System
The parasympathetic system relies on the binding of the neurotransmitter acetylcholine (Ach) to muscarinic receptors, and has various roles throughout the body.
Vasculature
Although blood vessels do express muscarinic receptors, they do not receive cholinergic innervation; however application of exogenous Ach results in a swift and profound vasodilation.
Heart
Activation of muscarinic receptors (M2-subtype) in the heart by Ach released from the vagus nerve causes a reduction in cardiac output via opposite effects to adrenergic stimulation: negative chronotropic effects and decreases in AV node conduction as well as decreasing the force of atrial contractions.
Platelet/Clotting System
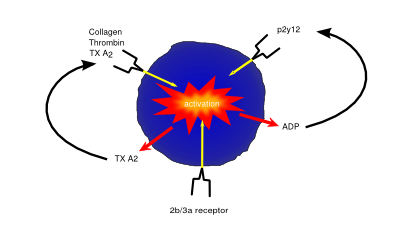
Platelets (also known as thrombocytes) are small cells lacking nuclei that are responsible for haemostasis, or blood clotting. Damage or injury leading to blood loss and exposure of extracellular collagen fibres is detected, activating platelets. Once activated, platelets become adhesive, sticking to both the damaged vessel wall and each other, forming a clump of cells, or ‘clot’, helping to dam the vessel leak. They then begin to secrete cytokines that encourage invasion of fibroblasts present in the surrounding tissue which form a more permanent patch, either by creating healthy tissue, or depositing extracellular matrix to form a scar.
There are several conditions in which abnormal clotting can be damaging to the body; excess clotting can lead to vascular blockage and ischaemia or stroke; less commonly, deficient clotting can lead to excess blood loss, for example in haemophilia. To combat these diseases, there are drugs that modulate the clotting process.
Anti-coagulants
Drugs that prevent clotting (anti-coagulants) are important in those with an increased risk of clotting-mediated damage such as a stroke or ischaemia.
As well being an analgesic and anti-pyretic, Aspirin is an anti-thrombotic agent given in low doses to those at risk of damage from clotting (e.g. following a heart attack). Aspirin’s anti-coagulant actions come from its suppression of key pro-clotting factors such as prostaglandins and thromboxanes via irreversible inactivation of the PTGS cyclooxygenase enzyme. This suppression of factors such as thromboxane A2 reduces platelet aggregation and thus prevents clot formation.
P2Y12 inhibitors such as clopidogrel exert their anti-coagulant effect via inhibition of the P2Y12 subtype of the platelet ADP receptor. By blocking P2Y12, these drugs prevent activation of platelets and the formation of the fibrin network needed for clotting.
Drugs such as abciximab and tirofiban prevent clotting via inhibition of the glycoprotein IIb/IIIa receptor preventing both platelet activation and aggregation.
Newer oral anticoagulants (NOACs), also referred to as direct oral anticoagulants (DOACs), include two classes based on their mechanism of action:
- **Direct thrombin inhibitors** (e.g. dabigatran) bind directly to thrombin (factor IIa), inhibiting its ability to convert fibrinogen into fibrin, thereby preventing the formation of fibrin clots.
- **Factor Xa inhibitors** (e.g. rivaroxaban, apixaban, edoxaban) selectively and reversibly inhibit factor Xa, a key enzyme in the coagulation cascade responsible for the conversion of prothrombin (factor II) to thrombin (factor IIa). By reducing thrombin generation, they effectively prevent thrombus formation.
NOACs are recommended by the European Society of Cardiology (ESC) as first-line therapy for stroke prevention in patients with atrial fibrillation, unless contraindicated. They have a predictable anticoagulant effect, rapid onset of action, and fewer drug-food interactions compared to vitamin K antagonists (e.g. warfarin).
Understanding the Cholesterol/LDL System in Cardiovascular Health
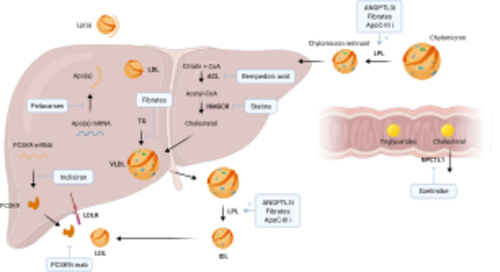
The cholesterol/LDL (low-density lipoprotein) system plays a pivotal role in cardiovascular health, acting as a key component in the development of atherosclerosis, a primary cause of cardiovascular diseases (CVD). This system's importance lies in its contribution to the transport of cholesterol, a vital lipid molecule, throughout the body. Cholesterol is essential for various biological functions, including the synthesis of cell membranes, hormones, and vitamin D. However, its management within the circulatory system is crucial to preventing adverse cardiovascular outcomes.
Cholesterol Transport and LDL's Role
Cholesterol travels through the bloodstream encapsulated within lipoproteins, which are particles made up of lipids and proteins. These lipoproteins are classified based on their density; low-density lipoproteins (LDL) and high-density lipoproteins (HDL) are among the most significant in terms of cardiovascular risk. LDL is often referred to as "bad" cholesterol because it contributes to the formation of plaque, a thick, hard deposit that can clog arteries and make them less flexible, a condition known as atherosclerosis. HDL, on the other hand, is known as "good" cholesterol because it helps remove cholesterol from the arteries, transporting it back to the liver for excretion or reuse.
LDL and Atherosclerosis
When LDL cholesterol levels are high, LDL particles can penetrate the endothelial lining of the arteries, becoming oxidized by free radicals. This oxidized LDL is recognized by the immune system as a foreign invader, attracting macrophages that ingest the LDL, transforming into foam cells. These foam cells accumulate to form fatty streaks, the earliest signs of atherosclerosis. Over time, these fatty streaks can develop into larger plaques, which can narrow the arteries and restrict blood flow. If a plaque ruptures, it can lead to the formation of a blood clot, which can cause a heart attack or stroke.
Regulation of Cholesterol/LDL Levels
The body's cholesterol levels are regulated by a complex interplay of synthesis, absorption, and excretion. The liver synthesizes cholesterol and secretes it into the bloodstream as part of very low-density lipoproteins (VLDL). As VLDL particles deliver their triglyceride content to cells, they become LDL particles. The liver also plays a key role in removing excess cholesterol from the blood, using receptors that bind to LDL particles and remove them from circulation.
Dietary intake of cholesterol and saturated fats can influence LDL levels, as can genetic factors, such as mutations in the LDL receptor gene, which can lead to familial hypercholesterolemia, a condition characterized by very high levels of LDL cholesterol and an increased risk of heart disease. Lifestyle factors, including diet, exercise, and smoking cessation, are primary interventions for managing LDL levels, alongside pharmacological treatments such as statins, which lower cholesterol levels by inhibiting its synthesis in the liver.
The Integral Role of LDL in Cardiovascular Health
Understanding the cholesterol/LDL system is essential for the prevention and management of cardiovascular diseases. By maintaining healthy levels of LDL cholesterol through lifestyle modifications and, when necessary, medication, individuals can significantly reduce their risk of developing atherosclerosis and its associated complications. This system's management is a cornerstone of cardiovascular health, underscoring the importance of regular monitoring and proactive interventions to maintain heart health and prevent disease.
To manage cardiovascular risk factors like serum lipids, various medication groups are essential:
- Statins (e.g., Atorvastatin, Simvastatin, Rosuvastatin, Pravastatin) lower cholesterol by inhibiting HMG-CoA reductase.
- Fibrates (e.g., Fenofibrate, Gemfibrozil) target triglycerides and increase HDL.
- PCSK9 inhibitors (e.g., Evolocumab, Alirocumab) significantly reduce LDL cholesterol by blocking PCSK9 protein.
- Bempedoic acid lowers LDL-C by inhibiting ATP citrate lyase.
These medications, alongside lifestyle modifications, form the cornerstone of cardiovascular disease prevention and management.
Pharmacokinetics
When administering drugs to a patient, it is crucial to know several facts about the drug in order to maximise efficacy and minimise side-effects/toxicity. These include information about what dose is effective, how long the drug remains active in the body, how quickly it is broken down/removed from the body, and how easily the body can absorb/use that drug. The following table details these pharmacokinetic properties and how they are calculated:
| Property | Description | Standard units (Abbreviation) | Formula |
|---|---|---|---|
| Dose | Amount of active drug given to patient | mg (D) | Drug Specific (From clinical studies) |
| Concentration | Amount of drug in a given plasma volume | µg/ml (C) | = D / Vd |
| EC50 | The concentration of drug needed to elicit a response halfway between zero and maximal responses. | µg/ml (EC50) | y = bottom + (Top-Bottom)/(1+ [x/EC50] Hill Coefficient) |
| Volume of Distribution | The theoretical volume the drug would occupy if distributed uniformly throughout the tissues to elicit the current plasma concentration. | L (Vd) | D / C |
| Elimination Constant (Rate) | The rate at which the drug is removed from the body. | h-1 (Ke) | ln(2) / t1/2 or CL / Vd |
| Bioavailability | How much of the administered dose is available for actual use by the body. | no units as expressing a fraction (f) | 100 × (AUC (po)×D (iv))/(AUC (iv)×D (po))
AUC = Area under curve po = oral administration iv = intravenous administration |
| Cmax or Cmin | The maximum (Cmax) / minimum (Cmin) plasma drug concentration reached following drug administration | µg/ml (Cmax or Cmin) | Identified via direct measurement of plasma C |
| tmax | The time it takes for a drug to reach Cmax following administration | h (tmax) | Identified via direct measurement of plasma C over time |
| Half-life | The time it takes for a drug to reach half its original concentration | h (t1/2) | ln(2) / Ke |
| Drug Clearance | The volume of plasma cleared of the drug over a set time | l/h (CL) | Vd x Ke or D / Area under curve |
Common Drug-Drug Interactions
It is important to be aware of the interactions that can occur between concomitantly administered drugs, as they may effect efficacy and/or toxicity, or produce adverse side effects. Such interactions could for example affect drug absorption, drug bioavailability or efficacy, or combine to produce unwanted metabolites, as well as possibly having effects on clinical analyses. If a combination of two drugs decreases the effect of one or both of them, the interaction is termed an antagonistic effect; however if, conversely, a combination of two drugs enhances the effect of one or both of them, the interaction is termed a synergistic effect. Drugs that act on the cardiovascular system are high in interactivity, which is an issue as cardiovascular patients normally receive more than one drug. Some common drug—drug interactions related to cardiovascular drugs are listed below:
| Drug | Drugs that ↑ drug action | Drugs that ↓ drug action |
|---|---|---|
| Digoxin |
|
|
| Warfarin |
|
|
| Clopidogrel |
|
|
| Furosemide |
| |
| ACE Inhibitors |
|
|
| ß-blockers |
|
|
| Statins |
|
|
There are several mechanisms by which drugs are broken down by the body, usually via degradation by enzymes. One common family of enzymes involved in drug metabolismis the cytochrome P450 (CYP) family; a large, diverse group of enzymes that encourage oxidation of a variety of substrates, both endogenous (e.g. steroid hormones) and exogenous (e.g. toxins and drugs). CYP enzymes account for up to 75% of drug metabolism, aiding some drugs to form their active compounds but mostly deactivating drugs into inactive metabolites to be excreted. CYP enzymes can influence drug actions in several ways; they can increase drug metabolism (either increasing action via formation of the active by-product or decreasing action by metabolism of the active drug) or their action can be inhibited by drugs that compete for access to the CYP enzymes active site, preventing the normal interaction between drug and enzyme. Many drugs exert their interactions with other drugs viainterference with the CYP system. For example, if Drug A is metabolised by CYP and Drug B inhibits CYP activity, co-administration will result in a decreased bioavailability of Drug A. In humans there are 18 families and 43 subfamilies of the CYP group of enzymes, which target different substrates. Some CYP enzymes important in cardiovascular medicine, their cardiovascular-drug substrates and some of their interactions are shown in the table below:
| Enzyme | Substrates (e.g.) | Inhibitors (e.g.) | Inducers (e.g.) |
|---|---|---|---|
| CYP2C19 |
|
|
|
| CYP3A4 |
|
|
|
| CYP2C9 |
|
|
|
| CYP2D6 |
|
|
|
In addition to drug-drug interactions, the actions of many drugs are also affected by food or drink. For example, care should be taken with alcohol consumption with many kinds of drugs, as it can put stress on the liver which is already working hard to metabolise drugs in the body. Grapefruit juice too can cause issues, as it is known to inhibit CYP3a. For more information of interactions between drugs and food/drinks see this guide: General Use of Medicine
Common Cardiovascular Drugs
| Drug Type | Examples (generic name) | Indications | Typical Dosage | Guidelines / Class of Indication | Side Effects (Prevalence %) |
|---|---|---|---|---|---|
| Anti-hypertensives | |||||
| Diuretics | Furosemide | Oedema | Furosemide: 20-40mg once daily | Mild gastro-intestinal disturbances, pancreatitis, hepatic encephalopathy, postural hypotension, temporary increase in serum-cholesterol and triglyceride concentration, hyperglycaemia, acute urinary retention, electrolyte disturbances, metabolic alkalosis, blood disorders, hyperuricaemia, visual disturbances, tinnitus and deafness, and hypersensitivity reactions (including rash, photosensitivity, and pruritus). | |
| Resistant Hypertension | Furosemide: 40-80mg once daily | Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IC Esc1 | |||
| ACE Inhibitors | Lisinopril, Captopril | Hypertension | Lisinopril: 10mg once daily initially, maintenance 20mg once daily | Hypertension: Class IA Esc2
Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IA Esc1 Hypertension in diabetics: Class IA Esc3 |
Hypotension (2.4%), renal impairment, persistent dry cough, angioedema, rash pancreatitis, upper respiratory-tract symptoms (2-10%), gastro-intestinal symptoms (1-2%), altered liver function tests, cholestatic jaundice, hepatitis, fulminant hepatic necrosis and failure, hyperkalaemia (2%), hypoglycaemia, blood disorders including thrombocytopenia, leucopenia, neutropenia, headache (3%), dizziness (2-12%), fatigue, malaise, taste disturbance, paraesthesia, bronchospasm, fever, serositis, vasculitis, myalgia (3%), arthralgia, positive antinuclear antibody, raised erythrocyte sedimentation rate, eosinophilia, leucocytosis, and photosensitivity. |
| Heart Failure | Lisinopril: 2.5-5mg once daily initially, maintenance 20-35mg once daily | Post STEMI: Class IA Esc4
Diabetic patients: Class IC Esc2 Symptomatic (NYHA class II-IV) HF: Class IA; Acute heart failure with ACS: Class IA Esc1 | |||
| Prophylaxis Following MI | Lisinopril: 5mg within 24 hours of onset, 5mg after 24 hours, then 10mg after 48 hours | ||||
| Diabetic nephropathy | Lisinopril: 10-20mg once daily | ||||
| Angiotensin Receptor Blockers | Losartan, Candesartan | Hypertension | Losartan: 50mg once daily | Hypertension: Class IA Esc2
Hypertension in diabetics: Class IA Esc3 |
Gastro-intestinal disturbances (<3%), dizziness (14%), angina, palpitation, oedema, dyspnoea, headache (14%), malaise, urticaria, pruritus, rash; |
| Left ventricular hypertrophy | Losartan: 12.5-150mg daily | LVH: Class IB Esc4 | |||
| Diabetic nephropathy | Losartan: 50mg daily | ||||
| Angiotensin Receptor Neprilysin Inhibitors | Sacubitril/Valsartan (Entresto) | Heart Failure with reduced ejection fraction (HFrEF) | 49/51mg twice daily initially; target dose 97/103mg twice daily | Symptomatic (NYHA class II-IV) HF: Class IA Esc1 | Hypotension (18%), renal dysfunction (3%), hyperkalemia (16%), cough, dizziness, angioedema (<1%), fatigue. Should not be used with ACE inhibitors (36-hour washout period required) or in pregnancy. |
| Heart Failure with mildly reduced or preserved ejection fraction | 49/51mg twice daily initially; target dose 97/103mg twice daily | HFmrEF and HFpEF: Class IA Esc1 | |||
| Alpha Blockers | Doxazosin, Prazosin | Hypertension | Doxazosin: 1mg once daily initially, increased to 4-8mg once daily | Drowsiness, hypotension (notably postural hypotension) (10-70% initially), syncope (1%), asthenia, dizziness, depression, headache (8-18%), dry mouth, gastro-intestinal disturbances, oedema, blurred vision (<5%), intra-operative floppy iris syndrome, rhinitis (<4%), erectile disorders (including priapism), tachycardia and palpitations (7-14%), gastrointestinal side-symptoms (4-5%), hypersensitivity reactions including rash, pruritus and angioedema. | |
| Congestive Heart Failure | Doxazosin: 4-16mg daily | ||||
| Raynaud's Syndrome | Doxazosin: 1-4mg daily | ||||
| Beta Blockers | Atenolol, Metoprolol, Propranolol | Hypertension | Atenolol: 25-50mg daily
Metoprolol: 100-200mg daily |
Gastro-intestinal disturbances (2-4%); bradycardia, heart failure, hypotension, conduction disorders, peripheral vasoconstriction, bronchospasm, dyspnoea; headache, fatigue, sleep disturbances (2-5%), paraesthesia, dizziness (2-5%), vertigo, psychoses; sexual dysfunction; purpura, thrombocytopenia; visual disturbances; exacerbation of psoriasis, alopecia; rarely rashes and dry eyes. | |
| Angina | Atenolol: 100mg once/twice daily
Metoprolol: 50-100mg twice daily |
ACS: Class IIaB Esc2
Angina in symptomatic (NYHA class II-IV) HF and LVD: Class IA Esc1 | |||
| Arrhythmias | Atenolol: 50-100mg daily
Metoprolol: 100-200mg daily |
Atrial fibrillation: Class IA; Polymorphic VT: Class IB Esc4
Symptomatic (NYHA class II-IV) HF, LVD and AF: Class IA; Management of VA in HF: Class IA Esc1 SVT: Class IIbC; Wide QRS-complex tachycardia of unknown origin: Class IIIC; Sinus tachycardia: Class IC; Poorly tolerated AVNRT with haemodynamic intolerance: Class IIaC; Recurrent symptomatic AVNRT: Class IC; Documented PSVT with only dual AV-nodal pathways or single echo beats demonstrated during electrophysiological study and no other identified cause of arrhythmia: Class IC; Infrequent, well tolerated AVNRT: Class IB; Focal junction tachycardia: Class IIaC; Nonparoxysmal junctional tachycardia: Class IIaC; WPW Syndrome: Class IIaC; AVRT, poorly tolerated: Class IIbC; Since or infrequent AVRT episode(s): Class IIaB; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IC; AF (Poorly tolerated): Class IIaC; AF (Stable flutter): Class IC; Prophylaxis of SVT during pregnancy: Class IIaB Acc5 | |||
| Calcium Channel Blockers | Nifedipine, Verapamil, Diltiazem | Hypertension | Nifedipine: 30-60mg once daily (slow-release) | Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IA Esc1 | Gastro-intestinal disturbance (2-11%); hypotension (1-5%), oedema (7-29%), vasodilatation, palpitation; headache (7-35%), dizziness (3-27%), lethargy (4-6%), asthenia (10-12%); less commonly tachycardia (<1-7%), syncope (<1%), chills, nasal congestion, dyspnoea (<3%), anxiety, sleep disturbance (<2%), vertigo (<3%), migraine, paraesthesia, tremor (1-8%), polyuria, dysuria, nocturia, erectile dysfunction (<2%), epistaxis, myalgia, joint swelling, visual disturbance (<2%), sweating (<2%), hypersensitivity reactions (<1%); rarely anorexia, gum hyperplasia, mood disturbances, hyperglycaemia, male infertility, purpura (<1%), and photosensitivity reactions (<1%); also reported dysphagia, intestinal obstruction, intestinal ulcer, bezoar formation, gynaecomastia, agranulocytosis, and anaphylaxis; |
| Raynaud's Syndrome | Nifedipine: 20-40mg once daily (slow-release) | ||||
| Angina (prophylaxis) | Nifedipine: 30-60mg once daily (slow-release) | Angina in symptomatic (NYHA class II-IV) HF and LVD: Class IIaA Esc1 | |||
| Heart Failure Medications | |||||
| Angiotensin Receptor Neprilysin Inhibitors | Sacubitril/Valsartan (Entresto) | Heart Failure with reduced ejection fraction (HFrEF) | 49/51mg twice daily initially; target dose 97/103mg twice daily | Symptomatic (NYHA class II-IV) HF: Class IA Esc1 | Hypotension (18%), renal dysfunction (3%), hyperkalemia (16%), cough, dizziness, angioedema (<1%), fatigue. Should not be used with ACE inhibitors (36-hour washout period required) or in pregnancy. |
| Heart Failure with mildly reduced or preserved ejection fraction | 49/51mg twice daily initially; target dose 97/103mg twice daily | HFmrEF and HFpEF: Class IA Esc1 | |||
| SGLT2 Inhibitors | Empagliflozin, Dapagliflozin, Canagliflozin, Sotagliflozin | Heart Failure with reduced ejection fraction | Empagliflozin: 10mg once daily
Dapagliflozin: 10mg once daily |
Symptomatic (NYHA class II-IV) HF with reduced EF: Class IA Esc1 | Genital mycotic infections (10%), urinary tract infections (4-8%), volume depletion (1-3%), hypotension, euglycemic diabetic ketoacidosis (<0.1%), acute kidney injury (2%), hypoglycemia (when used with insulin or insulin secretagogues), increased urination, thirst, and cholesterol levels. Rare serious adverse effects include Fournier's gangrene and lower limb amputations (particularly with canagliflozin). |
| Heart Failure with preserved ejection fraction | Empagliflozin: 10mg once daily
Dapagliflozin: 10mg once daily |
HFmrEF and HFpEF: Class I Esc1 | |||
| Type 2 Diabetes with high CV risk or CKD | Empagliflozin: 10-25mg once daily
Dapagliflozin: 10mg once daily Canagliflozin: 100-300mg once daily |
Type 2 diabetes with CV disease: Class IA Esc2
Type 2 diabetes with CKD: Class I Esc1 | |||
| Anti-Arrhythmics | |||||
| Class I (sodium channel blockers) | Flecainide, Lidocaine, Procainamide | Ventricular Arrhythmias | Flecainide: 50-100mg twice daily | Sustained VT and VF: Class IIbC Esc4
Pre-excited SVT/AF: Class IB; Wide QRS-complex tachycardia of unknown origin: Lidocaine (Class IIbB) / Procainamide (Class IB); Wide QRS-complex tachycardia of unknown origin with LVD: Class IB; Focal junction tachycardia: Class IIaC; WPW Syndrome: IIaC; AVRT, poorly tolerated: IIaC; Single or infrequent AVRT episode(s): Class IIbC; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IIaC; AF (Stable flutter): Class IIbA; Prophylaxis of SST during pregnancy: Class IIbB Acc5 |
Oedema, pro-arrhythmic effects (1-13%); dyspnoea; nervous-system side-effects including dizziness, asthenia, fatigue, fever; visual disturbances (13-28%); rarely pneumonitis, hallucinations, depression, confusion, amnesia, dyskinesia, convulsions, peripheral neuropathy; also reported gastro-intestinal disturbances (1-4%), anorexia, hepatic dysfunction, flushing, syncope, drowsiness, tremor, vertigo, headache, anxiety, insomnia, ataxia, paraesthesia, anaemia, leucopenia, thrombocytopenia, corneal deposits, tinnitus, increased antinuclear antibodies, hypersensitivity reactions (including rash, urticaria, and photosensitivity), increased sweating. |
| Class II (Beta blockers) | (See above) | (See above) | (See above) | (See above) | |
| Class III (Potassium channel blockers) | Amiodarone, Sotalol | Ventricular, Arrhythmias | Amiodarone: 200mg 2-3 times daily | Sustained VT and VF: Class IIaC; Polymorphic VT: Class IC Esc4
Management of VA in HF: Class IA; Prevention of VA in HF: Class IIbB Esc1 SVT: Class IIBC; Wide QRS-complex tachycardia of unknown origin: Class IB; Wide QRS-complex tachycardia of unknown origin with LVD: Class IB; Recurrent AVNRT unresponsive to beta blocker or calcium-channel blocker and patient not desiring RF ablation: Class IIbC; Focal junction tachycardia: Class IIaC; WPW Syndrome: IIaC; AVRT, poorly tolerated: Class IIaC; Since or infrequent AVRT episode(s): Class IIbB; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IIaC; AF (Poorly tolerated): Class IIbC; AF (Stable flutter): Class IIbC; Prophylaxis of SVT during pregnancy: Class IIIC Acc5 |
Gastro-intestinal disturbances (2-20%)), taste disturbances, hepatic disturbances (up to 50%); bradycardia; pulmonary toxicity (1-17%); tremor (9-59%), sleep disorders; hypothyroidism (5-10%), hyperthyroidism (5-10%); reversible corneal microdeposits (up to 98%); phototoxicity, persistent slate-grey skin discoloration (1-7%), injection-site reactions; less commonly onset or worsening of arrhythmia, conduction disturbances, peripheral neuropathy (1-105) and myopathy; very rarely sinus arrest, bronchospasm, ataxia (2-37%), benign intracranial hypertension, headache, vertigo, epididymo-orchitis, impotence, haemolytic or aplastic anaemia, thrombocytopenia, rash, hypersensitivity including photosensitivity (2-20%), anaphylaxis on rapid injection, hypotension (10-30%), respiratory distress syndrome, sweating, and hot flushes |
| Class IV (Calcium channel blockers) | (See above) | (See above) | (See above) | (See above) | |
| Digoxin | Supra-ventricular Arrhythmias | Acute: 0.75-1.5mg over 24 hours; Maintenance: 125-150µg daily | SVT: Class IIbC; WPW Syndrome: Class IIIC; AVRT, poorly tolerated: Class IIIC; Since or infrequent AVRT episode(s): Class IIIC; Prophylaxis of SVT during pregnancy: Class IC Acc5 | Gastro-intestinal disturbances (vomiting, diarrhoea, anorexia, abdominal pain) (25%); arrhythmias (up to 50%), AV conduction disturbances (50%); nervous system disturbances (dizziness, apathy, confusion, headache, fatigue, weakness) (25%); blurred or yellow vision; rash, eosinophilia, depression, anorexia, intestinal ischaemia and necrosis, psychosis, gynaecomastia on long-term use, and thrombocytopenia. | |
| Heart Failure | 62.5-125 µg daily | Symptomatic (NYHA class II-IV) HF: Class IIbB
Symptomatic (NYHA class II-IV) HF, LVD and AF: Class IB; Acute HF with AF and VT: Class IC Esc1 | |||
| Anti-platelet Drugs | |||||
| Aspirin | Prevention of thrombotic cerebro- or cardio-vascular disease | 75mg once/day | Prevention in AF: Class IC; Prevention in diabetic patients: IIaB Esc2
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA Esc1 Prevention in hypertensive patients with CV events: Class IA; Prevention in hypertensive patients without CV history but with reduced renal function/high risk: Class IIbA Esc3 Post-MI: Class Ia Esc3 |
Bronchospasm (10-30% in asthmatics); gastro-intestinal irritation (up to 83%), gastro-intestinal haemorrhage (occasionally major), also other haemorrhage (e.g. intracranial (0.5%), subconjunctival), chest pain (8.3%), oedema (4.5%), hypertension (4.3%). | |
| Pain / pyrexia | 300-600mg every 4-6 hours as necessary | ||||
| Clopidogrel | Prevention of thrombotic events (esp. when warfarin not tolerated) | 75mg once/day | Prevention in diabetic patients: IIaB; Primary and secondary prevention of stroke: Class IB Esc2
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA Esc1 Acute phase of coronary artery syndrome: Class IB; Non-cardioembolic cerebral ischaemic events: Class IA Esc3 |
Dyspepsia (5.2%), abdominal pain (5.6%), diarrhoea (4.5%); bleeding disorders including gastro-intestinal (2.0%) and intracranial (0.4%), nausea (3.4%), vomiting, gastritis, flatulence, constipation, gastric and duodenal ulcers, headache (7.6%), epistaxis (2.9%), dizziness (6.2%), paraesthesia, leucopenia, decreased platelets (very rarely severe thrombocytopenia), eosinophilia, rash (4.2%), pruritus (3.3%), vertigo, colitis, pancreatitis, hepatitis (<1%), acute liver failure, hypertension (4.3%), chest pain (8.3%), oedema (4.1%), vasculitis, confusion, hallucinations, taste disturbance, cough (3.9%), fatigue (4.8%) stomatitis, bronchospasm, interstitial pneumonitis, pyrexia (2.2%), blood disorders including thrombocytopenic purpura (5.3%), agranulocytosis, neutropenia (0.04%) and pancytopenia and hypersensitivity-like reactions (<0.1%)including fever, glomerulonephritis, arthralgia, Stevens-Johnson syndrome, toxic epidermal necrolysis, lichen planus. | |
| Acute myocardial infarction | 300mg daily initially then 75mg once/day | Post STEMI: Class IA Esc4 | |||
| Acute coronary syndrome | 300mg daily initially then 75mg once/day | ACS: Class IIaC Esc2 | |||
| Prasugrel | Prevention of thrombotic events. | 60mg bolus then 5-10mg once daily | Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA Esc1
Acute phase of coronary artery syndrome: Class IB Esc3 |
Haemorrhage (11.3%) (including gastro-intestinal (1.5%) and intracranial), haematoma, haematuria, hypertension (7.5%), hypotension (3.9%), headache (5.5%), back pain (5.0%), dyspnoea (4.9%), nausea (4.6%), dizziness (4.1%), cough (3.9%), fatigue (3.7%), chest pain (3.1%), arrhythmias including atrial fibrillation (2.9%) and bradycardia (2.9%), rash (2.8%), pyrexia (2.7%), oedema (2.7%), diarrhoea (2.3%), hypercholesterolaemia/hyperlipidaemia (7.5%), anaemia, rash,hypersensitivity reactions including angioedema (0.06%), thrombocytopenia (0.06%), thrombotic thrombocytopenic purpura. | |
| Ticragelor | Prevention of thrombotic events. | 180mg bolus then 90mg twice daily | Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA Esc1
Acute phase of coronary artery syndrome: Class IB Esc3 |
Dyspnoea (13.8%), haemorrhage, bruising; nausea (4.3%), vomiting, diarrhoea (3.7%), hypertension (3.8%), hypotension (3.2%), back pain (3.6%), abdominal pain, dyspepsia, gastritis, dizziness (4.5%), chest pain (3.7%), headache (6.5%), cough (4.9%), rash, pruritus, fatigue (3.2%), constipation, arrhythmias including atrial fibrillation (4.2%), paraesthesia, confusion, hyperuricaemia, raised serum creatinine (7.4%), vertigo. | |
| Vitamin K Antagonists | |||||
| Warfarin | Prevention of thrombotic/ embolic events (esp. after prosthetic valve insertion) | 5-10mg initially then tailored to individual (usually 3-9mg once daily at the same time) | Haemorrhage, nausea, vomiting, diarrhoea, jaundice, hepatic dysfunction, pancreatitis, pyrexia, alopecia, purpura, rash, 'purple toes', skin necrosis (increased risk in patients with protein C or protein S deficiency) | ||
| Acenocoumarol | Prevention of thrombotic/ embolic events (esp. after prosthetic valve insertion) | 4mg initially, followed by 1-8mg daily | Haemorrhage, nausea, vomiting, diarrhoea, jaundice, hepatic dysfunction, pancreatitis, pyrexia, alopecia, purpura, rash, 'purple toes', skin necrosis (increased risk in patients with protein C or protein S deficiency) | ||
| Non-vitamin K Antagonist Oral Anticoagulants (NOACs) | |||||
| Direct Thrombin Inhibitors | Dabigatran | Prevention of stroke and systemic embolism in non-valvular atrial fibrillation | 150mg twice daily (110mg twice daily if >80 years, GFR 30-50 mL/min, or increased bleeding risk) | Prevention of stroke in AF: Class IA Esc3 | Dyspepsia (10%), gastritis, esophagitis, gastroesophageal reflux (1-3%), abdominal pain (3%), diarrhea (3%), bleeding (including gastrointestinal 1-2%, intracranial <1%), hypersensitivity reactions (<0.1%), hepatic dysfunction. Highest renal elimination (80%) among NOACs; contraindicated if CrCl <30 mL/min. |
| Treatment and prevention of deep vein thrombosis and pulmonary embolism | 150mg twice daily after 5-10 days of parenteral anticoagulation | VTE treatment: Class IA Esc3 | |||
| Prevention of VTE after knee or hip replacement | 110mg 1-4 hours after surgery then 220mg once daily for 10 days (knee) or 28-35 days (hip) | VTE prophylaxis post-surgery: Class IA Esc3 | |||
| Factor Xa Inhibitors | Rivaroxaban, Apixaban, Edoxaban | Prevention of stroke and systemic embolism in non-valvular atrial fibrillation | Rivaroxaban: 20mg once daily with food (15mg if CrCl 15-49 mL/min)
Apixaban: 5mg twice daily (2.5mg twice daily if ≥2 of: age ≥80 years, weight ≤60kg, or serum creatinine ≥133μmol/L) Edoxaban: 60mg once daily (30mg if CrCl 15-50 mL/min or body weight ≤60kg) |
Prevention of stroke in AF: Class IA Esc3 | Bleeding (major 2-3%, clinically relevant non-major 4-7%), nausea (1-3%), anemia (1-2%), rash (1-2%), abnormal liver function tests (1-2%), thrombocytopenia (<1%).
Rivaroxaban and edoxaban must be taken with food. Varying degrees of renal elimination: edoxaban (50%), rivaroxaban (35%), apixaban (27%). Apixaban has lowest bleeding risk among NOACs. All contraindicated with CrCl <15 mL/min. |
| Treatment of deep vein thrombosis and pulmonary embolism | Rivaroxaban: 15mg twice daily for 21 days, then 20mg once daily
Apixaban: 10mg twice daily for 7 days, then 5mg twice daily Edoxaban: 60mg once daily after 5-10 days of parenteral anticoagulation |
VTE treatment: Class IA Esc3 | |||
| Prevention of recurrent deep vein thrombosis and pulmonary embolism | Rivaroxaban: 10-20mg once daily
Apixaban: 2.5mg twice daily Edoxaban: 60mg once daily (30mg if CrCl 15-50 mL/min or body weight ≤60kg) |
VTE recurrence prevention: Class IA Esc3 | |||
| Prevention of VTE after knee or hip replacement | Rivaroxaban: 10mg once daily for 2 weeks (knee) or 5 weeks (hip)
Apixaban: 2.5mg twice daily for 10-14 days (knee) or 32-38 days (hip) |
VTE prophylaxis post-surgery: Class IA Esc3 | |||
| Lipid-Lowering Drugs | |||||
| Statins | Simvastatin, Atorvastatin | Primary hyper-cholesterolaemia, combined hyperlipidaemia | Simvastatin: 10-20mg once daily | Dyslipidaemia: Class IA; Low HDL-C: Class IIbB; Elderly patients with CVD: IB; Elderly patients with no CVD but CV risk factors: IIbB; Type I diabetes: IC; Patients with CKD: IIaC; Transplant patients: Class IIaB; PAD: Class IA; HIV patients: IIaC Esc2
Hypertension in diabetics: Class IA; ACS: Class IA Esc3 |
Oedema (2.7%), abdominal pain (5.9%), nausea (5.4%), atrial fibrillation (5.7%), constipation (2.2%), gastritis (4.9%), diabetes mellitus (4.2%), myalgia (3.7%), headache (2.5%), insomnia (4.0%), vertigo (4.5%), bronchitis (6.6%), sinusitis (2.3%), eczema (4.5%), urinary tract infection (3.2%) |
| Homozygous Familial Hypercholesterolemia | Simvastatin: 40mg once daily | Homozygous Familial Hypercholesterolemia: Class I Esc2 | |||
| Secondary prevention in very high-risk patients | Atorvastatin: 40-80mg once daily | Very high-risk patients failing to achieve LDL-C goals on maximum tolerated statin: Class I Esc3 | |||
| PCSK9 Inhibitors | Evolocumab, Alirocumab, Inclisiran | Primary hypercholesterolemia, Mixed Dyslipidemia | Evolocumab: 140mg every 2 weeks or 420mg once monthly
Alirocumab: 75mg to 150mg every 2 weeks Inclisiran: 300mg initial dose, then 300mg at 3 months, followed by 300mg every 6 months |
Primary hypercholesterolemia and Mixed Dyslipidemia: Class I Esc2 | Injection site reactions (5.9%), nasopharyngitis (5.5%), upper respiratory tract infections (2.3%), flu (3.1%), back pain (3.2%), hypersensitivity reactions including rash, pruritus (1.7%), and rare cases of neurocognitive effects such as memory loss or confusion. Inclisiran has less frequent administration (twice yearly) as a small interfering RNA that inhibits PCSK9 production in the liver. |
| Homozygous Familial Hypercholesterolemia | Evolocumab: 420mg once monthly (may increase to 420mg every 2 weeks if needed) | Homozygous Familial Hypercholesterolemia: Class I Esc2 | |||
| Secondary prevention in very high-risk patients | Evolocumab: 140mg every 2 weeks
Alirocumab: 75mg to 150mg every 2 weeks Inclisiran: 300mg initial dose, then 300mg at 3 months, followed by 300mg every 6 months |
Very high-risk patients failing to achieve LDL-C goals on maximum tolerated statin: Class I Esc3 | |||
References
<biblio>
- Esc1 pmid=22611136
- Esc2 pmid=17220161
- Esc3 pmid=22555213
- Esc4 pmid=22922416
- Acc5 pmid=14557344
- Esc6 pmid=21712404
</biblio>