Physical Examination
The examination of the patient’s history is a critical in the evaluation of (suspected) heart disease. It includes information about the present complaints, development of symptoms over time, medical history and the patient's family. By combining this information, a chronology should be constructed in order to get a profound insight of the patient’s disease process. Determining what information is pivotal requires a detailed knowledge of the pathophysiology of cardiac disease. The time invested in carefully examining the patients’ history is well invested because this information is often very valuable in the diagnostic process.
History
The Present Illness
The history taking starts with a careful exploration of the patient’s symptoms currently and in the past. The exploration will consist of a thorough questioning of the frequency, intensity, severity, and duration of all patient reported symptoms. Also aggravating and relieving causes should be asked for. In addition to the patient reported symptoms, a strategic questioning of suspected symptoms can be added. Furthermore, current medications, including dosages, the frequency and administration, should be listed.
Antecedent Conditions
There are several systemic disease which may have cardiac involvement. Disease related to cardiac disease include, but are not limited to, are diabetes mellitus, cancer, thyroid disorders, inflammatory disease, and rheumatic fever. Prior treatments should be asked for because they could reveal the nature of the current problems and/or pre-existing disease.
Atherosclerotic Risk Factors
Atherosclerotic cardiovascular disease is the most prevalent type of heart disease in industrialized nations. The most important risk factors for atherosclerotic cardiovascular disease should be asked for are: family history of atherosclerotic disease (including age); diabetes mellitus; lipid disorders; hypertension; and smoking history.
Family History
A family history could provide important information not only in atherosclerotic cardiovascular disease but also for many other cardiac diseases. For example, congenital heart diseases are more common in the offspring of parents, family members or siblings. Genetic diseases, such as neuromuscular disorders, connective tissue disorders (eg, Marfan syndrome), lipid metabolism disorders can affect the cardiovascular system as well [1].
Common Symptoms
Chest Pain
Chest pain has a broad range of causes ranging from non-serious to serious to life threatening (Table 1). Chest pain is one of the important symptoms of ischemic heart disease. Furthermore, it is also associated with several other forms of heart disease. The most commonly used classification of stable angina is the CSS score (Table 2), and the Braunwald classification of unstable angina (Table 3)
| Table 1. Cause of Chest Pain |
|---|
Cardiovascular
|
Pulmonary
|
Gastrointestinal
|
Musculoskeletal
|
Psychological
|
Others
|
| Table 2. Canadian Cardiovascular Society Functional Classification of Angina Pectoris | ||
| Class | Definition | Specific Activity Scale |
|---|---|---|
| I | Ordinary physical activity (e.g., walking and climbing stairs) does not cause angina; angina occurs with strenuous, rapid, or prolonged exertion at work or recreation. | Ability to ski, play basketball, jog at 5 mph, or shovel snow without angina. |
| II | Slight limitation of ordinary activity. Angina occurs on walking or climbing stairs rapidly, walking uphill, walking or stair climbing after meals, in cold, in wind, or under emotional stress, or only during the few hours after awakening, when walking more than two blocks on level ground, or when climbing more than one flight of stairs at a normal pace and in normal conditions. | Ability to garden, rake, roller skate, walk at 4 mph on level ground, have sexual intercourse without stopping. |
| III | Marked limitation of ordinary physical activity. Angina occurs on walking one to two blocks on level ground or climbing one flight of stairs at a normal pace in normal conditions. | Ability to shower or dress without stopping, walk 2.5 mph, bowl, make a bed, play golf. |
| IV | Inability to perform any physical activity without discomfort. | Anginal symptoms may be present at rest. Inability to perform activities requiring 2 or fewer metabolic equivalents without angina. |
| Table 3. Braunwald Classification of Unstable Angina (UA) | ||||
| Severity | ||||
| Clinical Circumstances | ||||
| A | B | C | ||
| Develops in presence of extracardiac condition that intensifies myocardial ischemia (secondary UA) | Develops in the absence of extracardiac condition (primary UA) | Develops within 2 weeks after acute myocardial infarction (postinfarction UA) | ||
| I | New onset of severe angina or accelerated angina; no rest pain | IA | IB | IC |
| II | Angina at rest within past month but not within preceding 48 hr (angina at rest, subacute) | IIA | IIB | IIC |
| III | Angina at rest within 48 hr (angina at rest, acute) | IIIA | IIIB Troponin negative
IIIB Troponin positive |
IIIC |
| hr, hours; IAM, myocardial infarction; UA, unstable angina. | ||||
The five most common characteristics of ischemic chest pain are:
- The pain is classically deep, visceral, and intense. Patient often describe this pain as “pressing”, “tearing”, “constricting”, “burning”. Another common presentation is described as chest heaviness such as“a band across the chest”, or “weight in the centre of the chest”.
- The anginal pain is usually substernal located, but may extend to the left or right chest. Furthermore radiation of the pain is common, typically to the left shoulder and arm. Other locations are possible such as the neck, jaw, epigastrium, and, occasionally, the upper back.
- The duration of the pain is minutes, not seconds.
- The pain tends to be precipitated by exercise. The pain can also be provoked by heavy meals or emotional stress.
- The pain ablates promptly by resting (within minutes) or taking sublingual nitroglycerin.
| Table 4. Typical feature in various types of chest pain | ||||||
| Cause of pain | Type of pain | Referred pain | Response to posture/movement | Response to food/fluid | Tenderness | Response to nitroglycerin |
|---|---|---|---|---|---|---|
| Cardiovascular | ||||||
| Ischemic cardiac pain | Visceral | Yes | No | No | No | Yes |
| Aortic dissection | Visceral | Yes | No | No | No | No |
| Pericarditis | Visceral | Yes | Yes | No | No | No |
| Arrhythmia | Visceral | No | No | No | No | No |
| Pulmonary disease | Visceral/cutaneous | Usually no | No | No | No | No |
| Pneumothorax | Visceral/cutaneous | No | Yes | No | Usually no | No |
| Musculoskeletal | Cutaneous | No | Yes | No | Yes | No |
| Gastrointestinal | Visceral | Sometimes | No | Yes | No | Sometimes |
| Psychiatric | Visceral/cutaneous | No | No | No | No | No |
Dyspnoea
Dyspnoea is a frequent complaint of patients with a variety of cardiac diseases. Generally four types of dyspnoea can be distinguished:
- Exertional dyspnoea. Dyspnoea provoked by exercise, usually caused by a mild underlying condition because an increased demand of exertion is needed to precipitate symptoms.
- Dyspnoea at rest. Dyspnoea is suggestive for severe cardiac disease.
- Paroxysmal nocturnal dyspnoea. Dyspnoea characterized by the patient awakening after being asleep or recumbent for an hour or more. This type of dyspnoea is commonly caused by the redistribution of body fluids from the lower extremities into the vascular space and back to the heart, resulting in volume overload. Because of this pathophysiological background it suggests a more severe condition.
- Orthopnoea. Dyspnoea that occurs immediately on assuming the recumbent position. The mild increase in venous return (caused by lying down), before any fluid is mobilized from interstitial spaces in the lower extremities, is responsible for the symptom.
The cause of dyspnoea is certainly not limited to cardiac disease. Exertional dyspnoea, for example, can be due to pulmonary disease or anaemia. Orthopnoea is also a frequent complaint in patients with chronic obstructive pulmonary disease and postnasal drip. Resting dyspnoea is also a common sign of pulmonary disease such as pneumonia or bronchial infection. However, paroxysmal nocturnal dyspnoea is the most specific symptom for an underlying cardiac cause because alternative diagnoses are limited.
Syncope and Pre-syncope
Light-headedness, dizziness, pre-syncope, and syncope are important symptoms, often caused by a reduction in cerebral blood flow. The mentioned symptoms are nonspecific and can be due to a broad caused by a broad range of underlying pathophysiology such as metabolic conditions, dehydration, primary central nervous system disease, or inner-ear problems. Because bradyarrhythmias and tachyarrhythmias are important cardiac causes of these symptoms, they are of importance in the cardiovascular examination. A careful history taking, including preceding symptoms such as palpitations or chest pain, are of great importance. Further information on this topic can be found in [2].
Transient Central Nervous System Deficits
Transient central nerves system deficits such as transient ischemic attacks (TIAs) suggest the existence of (micro) emboli originating form greater vessels, the carotid arteries or the heart. Rarely a TIA can also be caused by emboli from the venous circulation through an intracardiac shunt. As a result, a TIA should prompt the search for underlying cardiovascular disease. Any sudden loss of blood flow to (parts of) the limbs are also suggestive for an underlying cardioembolic event.
Fluid Retention
Fluid retention is not a very specific symptom for heart disease but may be caused due to reduced cardiac function. Symptoms associated with fluid retention are peripheral oedema, weight gain, bloating, and/or abdominal pain from an enlarged liver or spleen. Decreased appetite, jaundice, nausea and vomiting can also occur from gut and hepatic dysfunction due to a build up of fluids.
Palpitation
Cardiac activity usually cannot be experienced by individuals in normal resting condition. If a patient is aware of its heart activity it is usually referred to as palpitation. Among cultures and patients there is no standard definition for the type of sensation represented by palpitation. It is often very illustrative to ask the patients to tap with their hand the perceived heartbeat. Most commonly palpitations are caused by an unusually forceful heart beat at a normal rate (60–100 bpm). When a patient senses more forceful contractions as usual without a significant increased heart rate, the palpitations are most commonly the result of endogenous catecholamine excretion. A customary cause of this phenomenon is anxiety. Another common experienced feeling is that of the heart stopping transiently and/or the occurrence of isolated forceful beats. The nature of this sensation is usually premature ventricular contractions. The rapid regular or irregular heart rates most linked to the term “palpitations” are the least common sensation reported by individual patients and is usually supraventricular of origin. More information on palpitations caused by arrhythmias can be found in the subsequent chapter [3].
Cough
Although cough is usually associated with disease of pulmonary origin, there are also several cardiac conditions that lead to pulmonary abnormalities which causes subsequent pulmonary disease and subsequent cough. A cough from cardiac origin is usually dry and non-productive. Pleural fluid retention from conditions such as heart failure or pulmonary hypertension from any cause may present as cough. Finally, it should be mentioned that angiotensin-converting enzyme inhibitors, which are frequently used in cardiac conditions, can cause a typical dry cough.
Physical Findings
Physical Examination - General inspection
The inspection of the patient is an observational task starting from the first contact all the way during the examination. All signs have to be carefully noticed because they can be of importance in the further diagnostic process.
General appearance in severe disease
- Signs of acute life threatening disease which need acute intervention to avert death. First important symptom is the colour of the face and skin which could be pallor or cyanotic as a sign of diminished circulation and/or respiration. Furthermore, the respiratory pattern and rate should be assessed for severe signs such as slow and irregular pattern, an audible rasp breathing with foam around the mouth, or in some cases a very fast and superficial breathing pattern. Additionally, the absence or a very weak pulsation could also be a sign of an acute life threatening disease. (For more detailed assessment of the pulse please notice the paragraph on this topic [4]) Be aware that these symptoms can be apparent, but there absence does not rule out an acute life threatening disease.
- Signs of severe diseases which are life threatening demands a similar approach with special attention to face colour, respiration and pulse. The face colour can be pallor, cyanotic or red. Respiration can be fast and superficial, often a sign of severe disease. The pulse is also important in case of severe disease, for more information see [5]. Furthermore it is important to assess signs of lowered degree of consciousness, emaciation, high body temperature and agitation.
Body position
Observe the patients’ position and if there is any preferred position in order to reduce or eliminate pain. For example, the pain of acute pericarditis is often minimized by sitting up, leaning forward, and breathing shallowly. When patients need to sit up right because of increasing dyspnoea when declining, so called orthopnoea, could be a sign of sever heart failure.
Consciousness
The consciousness state of the patient needs to be assessed to be assured of adequate brain functions or any disturbance. Subtle changes in consciousness are not easy to recognize and need special attention. Orientation in time, place and person need to be questioned. Gradual changes in consciousness can be very subtle, such changing conditions due to a delirium, or more apparent, such as somnolence or coma due to severe disease. In respect to heart disease we should be aware of these signs because they can relate to metabolic disease, such as hypoglycaemia in diabetes, and intoxication by medication or carbon monoxide.
Nutrient status
A lowered nutrient status can be a sign of several diseases. Generally there can be less intake, due to starvation, a lack of uptake, due to bowel disease, or a high combustion, due to chronic disease in for example metabolic disease or malign tumor growth. Emaciation, (extreme) low body weight, could be a sign of chronic heart failure or another systemic disorder (e.g., malignancy, infection).
Body composition
The body composition is a combination of skeletal build and soft tissue such as muscles and fat tissue. An abnormal body composition could be one of the signs of syndromes or non-cardiovascular conditions associated with cardiovascular abnormalities, such as for example Marfan syndrome, Down syndrome, Turner syndrome and ankylosing spondylitis [link congenital heart disease].
Vital signs
The vital signs, including height, weight, temperature, heart rate, blood pressure, respiratory pattern and rate, and peripheral oxygen saturation, can guide diagnosis and management in heart disease.
Height and weight
The height and weight permit the calculation of body mass index (BMI) and body surface area (BSA). Regular weight assessment is an important tool in the follow up of heart failure patients with significant fluid retention. Additionally, weight can be used for the adjustment of medication levels. Central obesity, as assed by the waist circumference (measured at the iliac crest) and waist-to-hip ratio (using the widest circumference around the buttocks), is a important determinant in the metabolic syndrome and one of the predictors of long-term cardiovascular risk.
Blood Pressure
One of the keystones of the cardiovascular physical examination is the proper measurement of the systemic arterial pressure by cuff sphygmomanometry at both arms. Blood pressure in the arterial system varies during the cardiac cycle, with a peak in the systole and the lowest level in diastole. The difference between the systolic and diastolic pressure is known as the pulse pressure. The arterial pressure is influenced by four important factors:
- Stroke volume of the left ventricle
- Compliance of the aorta and large arteries
- Resistance of the peripheral vasculature, particularly at the arteriolar level
- Blood volume and viscosity in the arterial system
A change in one or more of these factors alters systolic and/or diastolic pressure. The blood pressure fluctuates constantly and is influenced by a variety of factors such as pain, emotional state, physical activity, tobacco, coffee, drug use and consciousness. In order to measure a reproducible blood pressure several aspects have to been taken into account, most important summarized in Table 5.
| Table 5. Important aspects of blood pressure measurements |
|---|
|
The best way to measure the blood pressure starts with the palpation of the brachial artery, over which the diaphragm of the stethoscope has to be placed over it in the antecubital fossa. The onset and disappearance of the Korotkoff sounds define the systolic and diastolic pressures, respectively. There are some exceptions where the described assessment of the Korotkoff sounds is obsolete. For example, if the diastolic pressure drops near to zero, the point of muffling of the sounds is usually wrongly recorded as the diastolic pressure. The definition of hypertension dictates repeated measures under the same conditions, therefore the operator should record the arm (left/right) and the body position (lying/suspine/upright) of the patient to allow reproducible measurements to be made on serial visits. Normal and abnormal blood pressure levels in adult are represented in table 6. Please note that the normal levels in children are lower. The risk of cardiovascular disease increases progressively above 115/75 mmHg. Regarding hypotension, in daily practice blood pressure is considered too low only if noticeable symptoms are present.
Clinical trials have demonstrated that humans who maintain arterial pressures at the low end of these pressure ranges have much better long term cardiovascular health. Elevations in all age categories, although more commonly seen in elderly, are generally associated with increased morbidity and mortality.
| Table 6. Classification of blood pressure for adults | ||
| Category | systolic, mmHg | diastolic, mmHg |
|---|---|---|
| Hypotension | <90 | <60 |
| Desirable | 90–120 | 60–79 |
| Prehypertension | 121–139 | or 80–89 |
| Stage 1 Hypertension | 140–159 | or 90–99 |
| Stage 2 Hypertension | 160–179 | or 100–119 |
| Hypertensive Crisis | ≥ 180 | or ≥ 120 |
Measuring the blood pressure in a different position can be of clinical importance, for example to determine any orthostatic changes in blood pressure. In order to measure orthostatic hypotension the patient has to move from a supine to a standing position with a significant blood pressure drop within 3 minutes. Orthostatic hypotension is defined as a fall in blood pressure of more than 20 mm Hg systolic and/or more than 10 mm Hg diastolic. Orthostatic changes are of special interest in patients complaining of transient central nervous system symptoms, weakness, or unstable gait. Especially elderly have an increased tendency toward orthostatic hypotension due to a decreased compliance of the arteries and/or a decreased compensation mechanism increasing the stroke volume, resulting in a sudden drop of blood pressure which can cause dizziness or syncope. A difference in pressure between the right and left arm of 10 mmHg or more suggests an arterial obstruction in the arterial system of the arm were the lowest pressure is measured. Causes of significant differences between the blood pressure at the right an left arm are subclavian artery disease, aortic coarctation, supravalvular aortic stenosis or dissection. Additionally, in future assessment of the blood pressure the arm with the highest pressure should be measured. Furthermore, note that the pulse pressure is a crude measure of left ventricular stroke volume. A widened pulse pressure suggests that the stroke volume is large; a narrowed pressure, that the stroke volume is small. A widened pulse pressure also can be an indication of atherosclerotic disease, characterised by lengthened, more tortuous, and harder and less resistant arteries.
Pulse
The peripheral pulsations should be assed by both palpitation of the pulse and auscultation for bruits. Pulse abnormalities and bruits increase the likelihood of peripheral arterial disease. Pulsations should be assessed and documented in several arteries in the body in order to get an idea of the state of peripheral vasculature. Easily and fast palpable pulses in healthy individuals include the brachial, radial, and ulnar arteries of the upper extremities and the femoral, popliteal, dorsalis pedis, and posterior tibial arteries of the lower extremities. Minor or absent pulsations suggest a severe stenotic lesion proximal of the palpation site. To asses the cardiac (dys)function through the pulse generally an artery close to the heart should be selected, such as the carotid. Bounding high-amplitude carotid pulses suggest an increase in stroke volume and should be accompanied by a wide pulse pressure on the blood pressure measurement. A weak carotid pulse suggests a reduced stroke volume. [Figure 1]
[[Figure 1. Body locations for examining the pulse. source: homemade from http://commons.wikimedia.org/wiki/File%3ACirculatory_System_no_tags.svg]]
When examining the peripheral arterial pulsations by palpitation three important aspects should be noticed:
- The amplitude of the pulse, which correlates with the pulse pressure
- The contour of the pulse wave, depicted by the speed of up- and downstroke and the duration of its summit. In a physiological situation the upstroke is rapid, smooth and almost directly after the first heart sound. The summit is roughly midsystolic and has a smooth and rounded nature. The downstroke is generally less abrupt than the upstroke in a normal situation.
- Variations in the amplitude, either with respiration or from beat to beat.
The contour of the pulses depends on the stroke volume, ejection velocity, vascular capacity and compliance, and systemic resistance. The palpable pulsations reflect both the antegrade pulsatile flow of blood and reflection of the propagated pulse returning from the peripheral artery system. The arterial pulse upstroke increases with distance from the heart. In a physiological state, the percussion wave begins with systolic ejection (just after the first heart sound) and is the mainly monophasic pulse appreciated at the bedside. The dicrotic notch (an exaggerated, early, diastolic wave) demonstrates the aortic valve closure. A bounding pulse may occur in hyperkinetic states such as fever, anaemia, and thyrotoxicosis, or in pathologic states such as severe bradycardia, aortic regurgitation, or arteriovenous fistula. Two distinct pressure peaks in systole relate to a bifid pulse. This phenomenon is consistent with increased vascular compliance and may occur with fever or after exercise in a normal individual. With chronic severe aortic regurgitation, a large stroke volume ejected rapidly into a noncompliant arterial tree produces a reflected wave of sufficient amplitude to be palpated during systole. Hypertrophic obstructive cardiomyopathy can rarely produce a bifid systolic pulse with percussion and tidal (or reflected) waves. Usually the strength of the pulse is graded on a scale of 1 to 4, where 2 is a normal pulse amplitude, 3 or 4 is a hyperdynamic pulse, and 1 is a weak pulse. A low-amplitude, slow-rising pulse, which may be associated with a palpable vibration (thrill), suggests aortic stenosis. In patients over 70 years of age it is particularly important to palpate the abdominal aorta because abdominal aortic aneurysms are more prevalent.
A more than 10 mm Hg fall in systolic pressure with inspiration (pulsus paradoxus) is considered pathologic and a sign of pericardial or pulmonary disease, and can also occur in obesity and pregnancy without clinical disease. Pulsus paradoxus is measured by noting the difference between the systolic pressure at which the Korotkoff sounds are first heard (during expiration) and the systolic pressure at which the Korotkoff sounds are heard with each beat, independent of respiratory phase. Between these two pressures, the sounds are heard only intermittently (during expiration). In order to appreciate this subtle finding, the cuff pressure must be decreased slowly. Tachycardia, AF, and tachypnea make its assessment difficult. Pulsus paradoxus may also be palpable when the pressure difference exceeds 15 to 20 mm Hg. Pulsus paradoxus is not specific for pericardial tamponade and can also accompany massive pulmonary embolus, hemorrhagic shock, severe obstructive lung disease, or tension pneumothorax.
Pulsus alternans is defined by a beat-to-beat variability of the pulse amplitude. When only every other phase 1 Korotkoff sound is audible as the cuff pressure is slowly lowered, in a patient with a regular heart rhythm, independent of the respiratory cycle. Pulsus alternans is generally seen in severe heart failure. Important provoking factors of this phenomenon are severe aortic regurgitation, hypertension, and hypovolemic states. This phenomenon is attributed to cyclic changes in intracellular calcium levels and action potential duration. If it is the pulsus alternans is associated with electrocardiographic T wave the combination appears to increase arrhythmic risk.
Bruits often indicate accelerated blood flow velocity and/or flow disturbance at sites of arterial stenosis. By auscultation the supraclavicular and infraclavicular fossae should be assessed for evidence of subclavian artery stenosis. Additionally, the abdomen, flank, and pelvis should be examined with a stethoscope for evidence of stenosis in the aorta and its branch vessels; and each groin for evidence of femoral artery stenosis.
When patients suffer from aortoiliac disease, muscle atrophy in the legs may be seen. Other signs of chronic low-grade ischemia include thickened and brittle toenails, smooth and shiny skin, and subcutaneous fat atrophy of the digital pads. Patients with limb ischemia with dysfunction of the small arteries have a prolonged capillary refill of 3 seconds or more.
Jugular Venous Pulse
Assessment of the jugular venous pulse (JVP) is a method to assess central venous pressure and right atrium pressure. The examination can provide information about the patient’s volume status and cardiac function. Note that the JVP is not useful to asses in children younger than 12 years, because it is hard to asses in this age group.
Ideally the patient is positioned into the semi-upright posture, with an elevation of the head of the bed to 30°, that permits visualization of the top of the right internal jugular venous blood column. A venous arch may be used to measure the JVP more accurately. The JVP is the elevation at which the highest oscillation point of the jugular venous pulsations is usually seen in euvolemic patients. The height of the column of blood seen in the internal jugular vein, vertically from the sternal angle, is added to 5 cm of blood (the presumed distance to the centre of the right atrium from the sternal angle) to obtain an estimate of central venous pressure in centimetres of blood. In patients who are hypovolemic, you may anticipate that the jugular venous pressure will be low. Likewise, in hypervolemic patients, you may anticipate that the JVP will be high. As a result, in hypovolemic patients the head should be in a lowered position (up to 0°), while in a hypervolemic state the head should be subsequently raised of the bed.
Additionally, the characteristics of the right internal jugular pulse should be assessed, because they can be reveal clinical signs of right-heart function and rhythm disturbances. The distinctive waves of the jugular vein are summarized in Table 7 and visualized in Figure 2.
| Table 7. The jugular venous pulsation has a biphasic waveform. |
|---|
|
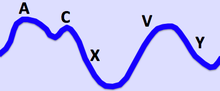
The a wave corresponds to right Atrial contraction and ends synchronously with the carotid artery pulse. The peak of the 'a' wave demarcates the end of atrial systole. The c wave corresponds to right ventricular Contraction causing the triCuspid valve to bulge towards the right atrium. The x descent follows the 'a' wave and corresponds to atrial relaXation and rapid atrial filling due to low pressure. The x'(x prime) descent follows the 'c' wave and occurs as a result of the right ventricle pulling the tricuspid valve downward during ventricular systole. The x' (x prime) descent can be used as a measure of right ventricle contractility. The v wave corresponds to Venous filling when the tricuspid valve is closed and venous pressure increases from venous return - this occurs during and following the carotid pulse. The " y " descent corresponds to the rapid emptYing of the atrium into the ventricle following the opening of the tricuspid valve. |
An elevated JVP is the classic sign of venous hypertension, typically in right sided heart failure. JVP elevation can be visualized as jugular venous distension, whereby the JVP is visualized at a level of the neck that is higher than normal. The paradoxical increase of the JVP with inspiration (instead of the expected decrease) is referred to as the Kussmaul sign, and indicates impaired filling of the right ventricle. The differential diagnosis of Kussmaul's sign includes constrictive pericarditis, restrictive cardiomyopathy, pericardial effusion, and severe right-sided heart failure.
| There are many JVP abnormalities which can be appreciated after careful assessment.
These abnormalities can be linked a broad spectrum of disease as highlighted below: |
|---|
Raised JVP, normal waveform
|
Raised JVP, absent pulsation
|
Large a wave (increased atrial contraction pressure)
|
Cannon 'a' wave (atria contracting against closed tricuspid valve)
|
Absent a wave (no univocal atrial depolarisation)
|
Large v wave (c-v wave)
|
Slow y descent
|
Parodoxical JVP (Kussmaul's sign: JVP rises with inspiration, drops with expiration)
|
Lungs
Evaluation of the lungs and respiration is an important part of the physical examination: Both diseases of the lung can affect the heart, as well as diseases of the heart can affect the lungs.
Auscultation and percussion is also a very important feature of the clinical examination of the lungs and respiratory system. The borders of the lung should be assessed by percussion. Furthermore percussion can reveal dullness, associated with for example abnormal pleural effusion. Several areas of the lungs should examined by auscultation in an upright position, if possible, both at the back and front of the patients, both base and apical fields covering all lung lobes.
During auscultation the patient’s respiratory pattern and rate should be assed. Tachypnoea is defined as increased breaths 20 or more breaths per minute. Physiological causes of tachypnoea are, for example, heightened oxygen demand due to exercise or labour during pregnancy. Amongst pathophysiological causes, tachypnoea can be a symptom of carbon monoxide poisoning, haemothorax or pneumothorax. Furthermore, pursing of the lips, a breathy quality to the voice, and an increased anteroposterior chest diameter would favour a pulmonary rather than cardiovascular cause of dyspnoea. Special attention is needed in case of so called Cheyne-Stokes respiration or Kussmaul breathing. Cheyne-Stokes is an abnormal pattern of breathing characterized by progressively deeper and sometimes faster breathing, followed by a gradual decrease that results in a temporary stop in breathing called an apnoea. These phenomena can occur during wakefulness or during sleep, where they are called the Central sleep apnoea syndrome. It may be caused by physiological abnormalities in chronic heart failure or damage to respiratory centres. Kussmaul breathing is defined as abnormally slow deep respiration characteristic of air hunger. This typical breathing pattern is caused by respiratory compensation for a metabolic acidosis, most commonly occurring in diabetics in diabetic ketoacidosis. The observation of respiration during sleep may reveal signs of disordered breathing associated with cardiovascular disease (e.g. obstructive sleep apnoea).
A clinical significant finding in the cardiovascular examination is rales at the pulmonary bases, indicating alveolar fluid collection. This fluid collection can be an important sign of heart failure in patients, however it is not always possible to distinguish rales caused by heart failure from those caused by pulmonary disease. Also the presence of pleural fluid, although useful in the diagnosis of heart failure, can be due to other causes. An additional combination of symptoms, dullness at the left base combined with bronchial breath sounds suggests an increase in heart size from pericardial effusion (Ewart sign) or cardiac enlargement due to another cause. Pathophysiological it is thought to be due to compression by the heart of a left lower lobe bronchus.
If severe right-heart failure develops or when venous return is restricted from entering the heart, venous pressure will increase in the abdomen, leading to hepatosplenomegaly and eventually ascites. These physical findings are not specific for heart disease, nonetheless they do help establish the diagnosis. Heart failure also leads to generalized fluid retention, usually manifested as lower extremity oedema. Oedema in de lower extremities is a sign of fluid retention, the skin can be compressed and will stay in this shape for a limited time. Oedema in lower extremities can also be caused by decreased venous return due to thrombosis or dysfunction of the venous system.
Assessment of the praecordium
Inspection
The praecordium is the front of the chest overlying the heart. First a global inspection should include the assessment shape and symmetry of the chest. A barrel chest could be a sign of chronic breathing problems. Also abnormalities of the shape of spine such as a kyphosis or scoliosis should be noticed, not only because of pathophysiological implications, but also demanding special care with regarding cardiac auscultation.
Scars could be found at different locations as signs of prior thoracic surgery. Most common locations include:
- Midline sternal scar: prior major thoracic surgery such as coronary artery bypass grafting and/or valve replacement
- Left sided thoracic scar (diagonal from under left breast to left axilla): Mitral valve surgery.
- Inferior of clavicula (left and/or right sided): Pacemaker / internal cardiac defibrillator implantation.
Above all, focus should be on visible cardiac pulsations, mostly in the apex region of the heart. In lean people the apex beat may be visible. A visible apex beat could also be a sign of abnormal conditions such as left ventricular aneurysm.
Palpitation
Palpitation of the praecordium should focus on the apex beat (defined as the most inferior point where the cardiac impulse is still palpable). The flat of your hand should be positioned so that the middle finger lies on the left 5th intercostal space of the patient, covering the anterolateral ribcage. The other fingers are positioned on the spaces above and below of the point of maximum pulsation. If no pulsation is felt, move the hand in all directions, feeling for a pulsation. Asking the patient to lean forward may help locate the apex beat if it is hard to palpate.
Several aspects of the apex beat should be noticed:
- Presence: In normal conditions the apex beat is palpable in the majority of patients.
- Location: The normal apex beat should be in the 5th intercostal space in the mid clavicular line.
- Size: The normal size of the apex beat has a diameter of about 3-4 cm in adults.
- Amplitude: The normal character of apex is a short pulsating beat.
- Duration: The duration of a normal apex beat is short during systole
- Thrills: A thrill is a palpable vibration caused by turbulent blood flow and is always pathological. Feel for a thrill (rather like a cat purring) at the apex, the upper part of the praecordium and in the sternal notch. The commonest cause of a thrill is aortic stenosis.
Presence of the apex beat
Several causes, both physiological and pathological, can be found for the absence of the apex beat. A mnemonic to remember these causes is DR POPE:
Physiological causes:
- Dextrocardia; the apex will be absent at the on the left side, but it will be present on the right side.
- Rib; if the apex beat is behind a rib it can not be found in an intercostal space. Turning the patient to the left lateral position will reveal the apex beat, confirming this cause.
Pathological causes:
- Pericardial effusion
- Obesity and thick chest wall
- Pleural effusion (left sided)
- Emphysema
Location of the apex beat
The location of the apex beat has to be assessed with respect to the tracheal position. If trachea is also shifted along with the displacement of apex beat, then it is due to mediastinal shift as a result of conditions such as lung fibrosis, collapse or pneumothorax.
If the trachea is central but the apex is displaced, the causes may be:
- Left ventricular enlargement - the apex will be displaced downwards and laterally.
- Right ventricular enlargement - the apex will displaced laterally.
- Cardiomegaly due to significant enlargement of other chambers can also cause displacement
- Pectus excavatum – resulting in a different location due to congenital thoracic deformity
Abnormal types (Size, amplitude, duration) of apex beat:
- Tapping Apex - This is a close to normal apex beat regarding to size, duration and amplitude with a palpable first heart sound. The palpable first heart sound is mostly caused by mitral stenosis.
- Hyperdynamic Apex – The amplitude and duration of the apex beat is forceful but ill-sustained and is palpable over a larger area size than normal (diffuse). A hyperdynamic apex beat is classically seen in ventricular dilatation due to volume overload conditions (aortic regurgitation, hyperdynamic circulation etc).
- Heaving Apex – The amplitude of this apex beat is a forceful, the duration sustained and dislocated. Classically seen in ventricular hypertrophy due to pressure overload conditions (aortic stenosis, systemic hypertension etc).
- Double Impulse Apex - Two impulses felt during systole rather than the normal single upstroke. This apex beat characteristic can be observed in hypertrophic cardiomyopathy
- Dyskinetic Apex - An uncoordinated apex beat can be seen due to dyskinetic movements in the infarcted myocardium.
- Left parasternal impulse or “heave” – An apex beat felt at the left side of the sternum due to right ventricular enlargement
Cardiac Auscultation
The acceleration and deceleration of blood and the subsequent vibration of the cardiac structures during the phases of the cardiac cycle are causing heart sounds. In healthy adults, there are two normal heart sounds often described as a lub and a dub (or dup), that occur in sequence with each heart beat. These are the first heart sound (S1) and second heart sound (S2), produced by the closing of the atroventricular valves and semilunar valves respectively. In addition to these normal sounds, a variety of other sounds may be present including heart murmurs, adventitious sounds, and gallop rhythms S3 and S4.To hear cardiac sounds, use a stethoscope with a bell and a tight diaphragm. The bell is best used to hear low-frequency sounds which are associated with ventricular filling. The diaphragm is best used to appreciate the medium-frequency sounds that are associated with valve opening and closing. Cardiac murmurs are caused due to turbulent blood flow and are usually high-to-medium frequency. In most cases the diaphragm is best used to hear cardiac murmurs. An important exception to this is the low-frequency atrioventricular valve inflow murmurs, such as that produced by mitral stenosis, which are best heard with the bell. Murmurs may be physiological or pathological. Abnormal murmurs can be caused by stenosis restricting the opening of a heart valve, resulting in turbulence as blood flows through it. Abnormal murmurs may also occur with valvular insufficiency (or regurgitation), which allows backflow of blood when the incompetent valve closes with only partial effectiveness.
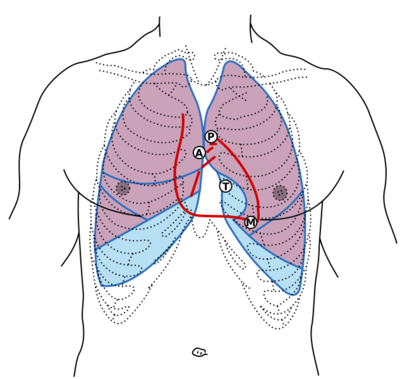
Auscultation should take place in areas that correspond to the location of the heart and great vessels. Such placement will, of course, need to be modified for patients with unusual body habitus or an unusual cardiac position. When no cardiac sounds can be heard over the precordium, they can often be heard in either the subxiphoid area or the right supraclavicular area. The body positions for the placement of the stethoscope are shown in Figure 3.
Auscultation in various positions is recommended to appreciate sounds and murmurs at maximally. For the first examination the patient should be in a suspine position. Furthermore, the patient should be asked to roll partly onto the left side into the left lateral decubitus position. This position brings the left ventricle close to the chest wall. In this position accentuates or brings out a left-side S3 and S4 an mitral murmurs. The other important position is sitting and forward leaning. The patient should be asked to completely exhale and stop breathing in expiration. The stethoscope diaphragm should be pressed along the left sternal border and at the apex. This position accentuates or brings out aortic murmurs maximally.
S1
The first heart tone, or S1, is composed of components mitral (M1) and tricuspid valve closure (T1). Normally the sound of mitral valve closure precedes valve closure slightly. The first heart tone is caused by the sudden block of reverse blood flow due to closure of the atrioventricular valves, i.e. tricuspid and mitral (bicuspid), at the beginning of ventricular contraction, or systole. The papillary muscles in each ventricle start with contraction parallel to the ventricles. The papillary muscles are attached to the tricuspid and mitral valves via chordae tendineae, which hold the cusps or leaflets of the valve to a closed position. During systole the chordae tendineae prevent the valves from blowing into the atria. The closing of the inlet valves prevents regurgitation of blood from the ventricles back into the atria. The S1 sound results from reverberation within the blood associated with the sudden block of flow reversal by the valves. If T1 occurs slightly after M1, then the patient likely has a dysfunction of conduction of the right side of the heart such as a right bundle branch block.
S2
The second heart tone, or S2, is composed of components the closure of the aortic (A2) an pulmonary valve (P2). Normally A2 precedes P2 especially during inspiration when a split of S2 can be heard in normal conditions. It is caused by the sudden block of reversing blood flow due to closure of the aortic and pulmonary valve at the end of ventricular systole, i.e. beginning of ventricular diastole. As the left ventricle empties, its pressure falls below the pressure in the aorta. Aortic blood flow quickly reverses back toward the left ventricle, catching the pocket-like cusps of the aortic valve, and is stopped by aortic (outlet) valve closure. Similarly, as the pressure in the right ventricle falls below the pressure in the pulmonary artery, the pulmonary (outlet) valve closes. The S2 sound results from reverberation within the blood associated with the sudden block of flow reversal.
Splitting of S2, also known as physiological split, normally occurs during inspiration because the decrease in intrathoracic pressure increases the time needed for pulmonary pressure to exceed that of the right ventricular pressure. A widely split S2 can be associated with several different cardiovascular conditions, including right bundle branch block and pulmonary stenosis.
Extra heart sounds
The rarer extra heart sounds are heard in both normal and abnormal situations. The presence of a extra heart sound is also referred to as a gallop rhythm, because together with the normal heart tones the additional heart sounds resemble this typical sound.
S3
The third heart sound also called a protodiastolic gallop or ventricular gallop. The third heart sound occurs at the beginning of diastole after S2 and is lower in pitch than S1 or S2 as it is not of valvular origin. S3 is thought to be caused by the oscillation of blood back and forth between the walls of the ventricles initiated by inrushing blood from the atria. The reason the third heart sound does not occur until the middle third of diastole is probably that during the early part of diastole, the ventricles are not filled sufficiently to create enough tension for reverberation.
The third heart sound is best heard with the bell-side of the stethoscope (used for lower frequency sounds). A left-sided S3 is best heard in the left lateral decubitus position and at the apex of the heart, which is normally located in the 5th left intercostal space at the midclavicular line. A right-sided S3 is best heard at the lower-left sternal border. The way to distinguish between a left and right-sided S3 is to observe whether it increases in intensity with inspiration or expiration. A right-sided S3 will increase on inspiration whereas a left-sided S3 will increase on expiration.
The third heart sound is benign in youth, some trained athletes, and sometimes in pregnancy. If the sound re-emerges later in life it may be caused by pathological diseases conditions such as a failing left ventricle as in dilated congestive heart failure. The third heart sound may also be a result of tensing of the chordae tendineae during rapid filling and expansion of the ventricle due to volume overload.
S4
The fourth heart sound when audible in an adult is called a pre-systolic gallop or atrial gallop. This gallop is produced by the sound of blood being forced into a stiff and/or hypertrophic ventricle.
The sound occurs just after atrial contraction at the end of diastole and immediately before S1. It is best heard at the cardiac apex with the patient in the left lateral decubitus position and holding his breath.
The pathological fourth heart sound is a sing of a failing left ventricle or other pathological conditions such as restrictive cardiomyopathy.
The combined presence of S3 and S4 is a quadruple gallop. At rapid heart rates, S3 and S4 may merge to produce a summation gallop.
Murmurs
Heart murmurs are produced as a result of turbulent flow of blood. They are usually heard as a whooshing sound. The term murmur only refers to a sound believed to originate within blood flow through or near the heart associated structures. In order to produce an audible noise rapid blood velocity is necessary. It is important to keep in mind that most heart problems do not produce any murmur and most valve problems also do not produce an audible murmur.
Traditionally, the origin of heart murmurs can be based on six important factors:
- Location – The hearth sounds can be typically best heard in the following areas:
- Aortic in the parasternal second right intercostal space
- Pulmonic in the parasternal second left intercostal space
- Tricuspid area in the parasternal fourth left intercostal space
- The mitral area in the midclavicular fifth left intercostal space.
Keep in mind that due to many diseases the position of the heart can be altered.
- Intensity – The intensity of murmurs has a wide range. In general the lower intensity is associated with more benign conditions, while higher intensities are associated with pathological conditions. However, keep in mind that a lot of diseases do not produce any heart murmur and valvular diseases do not always produce audible murmurs. The intensity of the murmur can be classified according to Table 8.
| Table 8. Graduation of Murmurs | |
| Grade | Description |
|---|---|
| Grade 1 | Very faint, heard only after listener has "tuned in"; may not be heard in all positions. Only heard if the patient "bears down" or performs the Valsalva manoeuvre. |
| Grade 2 | Quiet, but heard immediately after placing the stethoscope on the chest. |
| Grade 3 | Moderately loud. |
| Grade 4 | Loud, with palpable thrill (i.e., a tremor or vibration felt on palpation) |
| Grade 5 | Very loud, with thrill. May be heard when stethoscope is partly off the chest. |
| Grade 6 | Very loud, with thrill. May be heard with stethoscope entirely off the chest. |
- Timing in the cardiac cycle – Murmurs could be heard early, mid or late in systole, throughout systole (holosystolic), early, mid or late in diastole or continuous. See below for more detail.
- Shape – Several shapes can be distinguished as shown in Figure 4.
[[Figure 4. Murmur sound shapes. Source: homemade]]
- Radiation – Some of the underlying pathologic disease cause murmurs to radiate. The distinctive pattern follows the blood flow from the point of maximal intensity:
- Aortic regurgitation – From the aortic valve area into the apex
- Aortic stenosis – From the aortic valve area into the carotid arteries
- Hypertrophic cardiomyopathy – From the tricuspid area into to the aortic area
- Mitral regurgitation – From the mitral area into the left axilla.
- Response to manoeuvres:
- Normal respiration – The patient should be in a suspine position and breathing normally. In general right-sided cardiac murmurs should increase in intensity with normal inspiration. The increasing intensity during inspiration is caused due to the reductions in intrathoracic pressure that increase venous return from the abdomen and the head, leading to an increased flow through the right heart chambers. The consequent increase in pressure increases the intensity of right-sided murmurs.
- Sitting and leaning forward - Changes in position are an important part of normal auscultation and can also be of great value in determining the origin of cardiac murmurs. Murmurs dependent on venous return, such as innocent flow murmurs, are softer or absent in upright positions. Murmurs associated with hypertrophic obstructive cardiomyopathy, are accentuated by reduced left ventricular volume associated with the upright position.
- Rapid squat from the standing position – The rapid change of body position increases venous return and left ventricular volume and therefore accentuates flow murmurs but diminishes the murmur of hypertrophic obstructive cardiomyopathie. The stand-squat manoeuvre is also useful for altering the timing of the midsystolic click caused by mitral valve prolapse during systole. When the ventricle is small during standing, the prolapse occurs earlier in systole, moving the midsystolic click to early systole. During squatting, the ventricle dilates and the prolapse is delayed in systole, resulting in a late midsystolic click.
- Lateral decubitis – In this manoeuvre the patient rolls partly onto the left side and the apex should be auscultated. By bringing the left ventricle closer tot the chest, left-sided murmurs an generally louder.
- Vasalva manoeuvre – During this manoeuvre the patient bears down and expires against a closed glottis (hold the breathe and strain hard for 10 seconds). A increasing intrathoracic pressure and markedly reducing venous return to the heart will be caused by this manoeuvre. Almost all cardiac murmurs decrease in intensity, however during strain the murmur of hypertrophic obstructive cardiomyopathy may become louder because and the murmur associated with mitral regurgitation from mitral valve prolapse may become longer and louder because of the earlier occurrence of prolapse during systole. After release of the strain the murmur will decrease and most other murmurs will increase in intensity.
- Isometric hand grip – The patient has to relax his body while squeezing both fists. The manoeuvre increases arterial and left ventricular pressure, thus increasing afterload and flow gradient for mitral regurgitation, ventricular septal defect, and aortic regurgitation; the murmurs should then increase in intensity. The manoeuvre is in particular useful in distinguishing the increasing murmur of mitral regurgitation from a similar or lowering pitch aortic stenosis.
Murmurs categorized by time in cardiac cycle
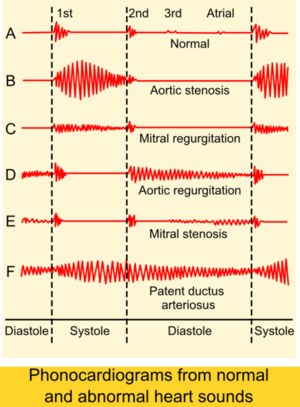
A schematic scheme of the heart sounds and heart murmurs are shown in Figure 5.
Systolic murmurs are very common and do not always imply cardiac disease. Most murmurs fall in the 1–3 audible intensity range, however murmurs in the 4–6 range are almost always due to pathologic conditions. Again, severe disease can exist with grades 1–3 or no cardiac murmurs. Distinguishing benign from pathologic systolic flow murmurs is one of the major challenges of clinical cardiology. Benign flow murmurs are heard in 80% of children with a declining incidence with increasing age. Other physical conditions known for benign heart systolic murmurs are pregnancy or thin adults or athletic adults. The murmur is usually benign in a patient with a soft flow murmur that diminishes in intensity in the sitting position and neither a history of cardiovascular disease nor other cardiac findings. The physiological flow murmurs are usually heard in grades 1–2 and occur very early in systole. These murmurs have a vibratory quality and are usually less apparent when the patient is in the sitting position (when venous return is less). If an ejection sound is heard, there is usually some abnormality of the aortic or pulmonary valve. The most common systolic murmur is the becoming stronger and fading (crescendo/decrescendo) murmur. This murmur increases in intensity as blood flows early in systole and diminishes in intensity through the second half of systole. This murmur can be caused by a strong flow in a normal heart or to obstructions of flow, as occurs with a stenotic semilunar valve, or hypertrophic cardiomyopathy.
The holosystolic, or pansystolic, murmur is almost always associated with cardiac pathology. The most common cause of this murmur is atrioventricular valve regurgitation. A holosystolic murmur can also be heard in conditions such as ventricular septal defect, in which an abnormal communication exists between two chambers of markedly different systolic pressures. Although it is relatively easy to determine that these murmurs represent an abnormality, it is more of a challenge to determine their origins. Conditions such as mitral regurgitation, which usually produce holosystolic murmurs, may produce crescendo/decrescendo murmurs, adding to the difficulty in differentiating benign from pathologic systolic flow murmurs.
Diastolic murmurs are always abnormal. The most frequently heard diastolic murmur is the high-frequency decrescendo early diastolic murmur of aortic regurgitation. This murmur however needs careful listening because it can be hard to hear due to its high frequency. This murmur is usually heard best at the upper left sternal border or in the aortic area (upper right sternal border) and may radiate to the lower left sternal border and the apex. Although the murmur of pulmonic regurgitation may sound like that of aortic regurgitation when pulmonary artery pressures are high, it is usually best heard in the in a different location. The pulmonic area in the left second intercostal space parasternally is usually the best place to appreciate the murmur. If structural disease of the valve is present with normal pulmonary pressures, the murmur usually has a midrange frequency and begins with a slight delay after the pulmonic second heart sound. Mitral stenosis produces a low-frequency rumbling diastolic murmur that is decrescendo in early diastole, but may become crescendo up to the first heart sound with moderately severe mitral stenosis and sinus rhythm. The murmur is best heard at the apex in the left lateral decubitus position with the bell of the stethoscope. Similar findings are heard in tricuspid stenosis, but the murmur is loudest at a different location, the lower left sternal border.
A continuous murmur implies a connection between a high- and a low-pressure chamber throughout the cardiac cycle, such as occurs with a fistula between the aorta and the pulmonary artery. If the connection is a congenital patent ductus arteriosus, the murmur is heard best under the left clavicle; it has a machine-like quality. The most challenging is to distinguish the continuous murmurs from the combination of systolic and diastolic murmurs in patients with combined valve disease (eg, aortic stenosis and regurgitation).