Myocardial and Pericardial Disease: Difference between revisions
No edit summary |
|||
| Line 445: | Line 445: | ||
Treatment of cardiac dysfunction is treated according to the nature of cardiac involvement. Conduction disorders may present which require pacing, and standard heart failure therapy may be instituted in case of ventricular dilatation and functional impairment. Ventricular tachyarrhythmia may be found in particular in myotonic dystrophia, and require the implantation of an internal cardiac defibrillator to prevent its associated sudden cardiac death. | Treatment of cardiac dysfunction is treated according to the nature of cardiac involvement. Conduction disorders may present which require pacing, and standard heart failure therapy may be instituted in case of ventricular dilatation and functional impairment. Ventricular tachyarrhythmia may be found in particular in myotonic dystrophia, and require the implantation of an internal cardiac defibrillator to prevent its associated sudden cardiac death. | ||
== Pericardial Disease == | |||
The pericardium comprises two layers; the visceral layer that adheres to epicardial surface of the heart, and the parietal layer that surrounds most of the heart. Pericardial disease is common, and diagnosis is usually straightforward as the pericardium reacts to disruption by a wide variety of agents and processes in a relatively uniform manner. Typical presentation is with chest pain and fever, production of pericardial fluid with possible cardiac tamponade, or a constrictive pattern by thickening, retraction and calcification. | |||
=== Acute pericardial syndromes === | |||
==== Cardiac Tamponade ==== | |||
An increase in intrapericardial pressure, resulting in compression of the heart, and thereby a restriction of cardiac inflow, is termed cardiac tamponade. Tamponade may result from pericardial effusion of any cause. The intrapericardial pressure importantly determines to what extent cardiac inflow is decreased, but two factors need to be taken into consideration. First, intrapericardial pressure is determined not only on the amount of fluid that accumulates, but also on the rate with which this accumulation proceeds, and the available distensibility of the pericardium. Chronic effusions may therefore lead to small increases in intrapericardial pressures in the presence of large fluid accumulations, and small accumulations may directly lead to sever cardiac tamponade for example in free wall rupture. Second, the intravascular volume and intra-atrial, and -ventricular pressures determine at what pressure inflow becomes impaired. When intrapericardial pressure exceeds right atrial pressure (approximately 8 mmHg), tamponade usually follows. However, in patients in whom intra-atrial pressure is decreased, for example due to volume depletion, tamponade may occur at low intrapericardial pressures; low-pressure cardiac tamponade. | |||
With increasing intrapericardial pressure, clinical features increase with severe hemodynamic compromise as its end stage. First, small changes in intrapericardial pressure induce subtle changes in arterial pressure, cardiac output, and variations in arterial pressure with inspiration (pulsus paradoxus). When intrapericardial pressure reaches levels similar to right atrial and diastolic right ventricular pressure echocardiographic evidence of tamponade may be found in diastolic collapsing of the right atrium, and increased variations of blood flow velocity over the cardiac valves with respiration. Fulminant clinical tamponade presents with symptoms that depend on its etiology. Acute cardiac tamponade based upon aortic of free wall rupture presents with syncope and sudden collapse, whereas tamponade in the setting of an acute inflammatory pericardits may present with pericardial chest pain, and dyspnoea. Therefore, in patients presenting with chest discomfort, dyspnoea, tachycardia or tachypnoea cardiac tamponade should be suspected when jugular distension, hypotension or pulsus paradoxus is present. Auscultation may reveal pericardial friction rub, and heart sounds may be faint. Echocardiography shows pericardial effusion, the previously mention diastolic collapsing of the cardiac cavities, increased flow velocity over tricuspid and pulmonary vales, and decreased flow velocity over the aortic and mitral valves. | |||
When secondary to inflammatory pericarditis, up to moderate tamponade may be treated by anti-inflammatory drugs. In severe tamponade, pericardiocentesis should be performed to immediately alleviate intrapericardial pressure. Surgical drainage should be considered when pericardiocentesis is unsuccessful, or when tamponade recurs. | |||
==== Acute pericarditis ==== | |||
{| class="wikitable" border="0" style='float: right' | |||
|- align='left' | |||
!Table Causes of acute pericarditis<br /> | |||
|- align='left' | |||
| Acute idiopathic pericarditis | |||
|- align='left' | |||
| Infectious pericarditis: | |||
* Viral | |||
* Tuberculosis | |||
* Bacterial | |||
* Others | |||
|- align='left' | |||
| Postpericardiotomy syndrome | |||
|- align='left' | |||
| Postmyocardial infarction pericarditis | |||
|- align='left' | |||
| Renal insufficiency | |||
|- align='left' | |||
| Neoplastic disease | |||
|- align='left' | |||
| Chest trauma | |||
|- align='left' | |||
| Irradiation | |||
|- align='left' | |||
| Collagen diseases | |||
|} | |||
Acute inflammation of the pericardium may result from a wide variety of etiologies TABLE, and typically presents with chest pain, a pericardial friction rub on auscultation, and repolarization changes on the electrocardiogram. | |||
Patients present with a rapid-onset chest pain syndrome, located precordial and retrosternal, and radiating to the subclavian region, the back and the trapezoid region. Chest pain is of moderate severity, lasting for several days, and increases with inspiration or chest movement. Patients typically alleviate the pain by sitting, and leaning forward. | |||
A pericardial friction rub is pathognomonic of pericarditis, and ECG changes are frequently present, which comprise diffuse concave ST-segment elevation, with positive T-waves in several leads. Atrial injury is accompanied by PR-segment depression. After several hours to a few days, ST-segments return iso-electric, and negative T-waves may occurs subsequently and may persist for several weeks, although they frequently normalize within days. Pericardial effusion may be present, and is diagnosed by chest X-ray when fluid accumulation exceeds 250mL, or by echocardiography. | |||
Initial presentation may therefore mimick ST-segment elevation myocardial infarction. Onset of chest is, however, less abrupt in acute pericarditis, and varies with respiration. Diffuse ST-segment changes are present in pericarditis, whereas STEMI presents with ST-segment elevation, and reciprocal depression, in leads corresponding to the ischemic myocardium. Biomarkers may, however, be positive in both syndromes. | |||
Treatment consists of aspirin while pain and fever are present, which usually adequately alleviates symptoms. Another option is NSAIDs, which are recommended when aspirin in insufficient or contraindicated. Corticosteroids should, however, be avoided as they are associated with relapsing pericarditis. Hospital admission may be necessary in patients with high fever, large effusions or cardiac tamponade. | |||
==== Recurrent pericarditis ==== | |||
In 8 up to 80% of patients, pericarditis recurs after a first episode of acute pericarditis. A continuous type, in which symptoms recur shortly after cessation of anti-inflammatory therapy, and an intermittent type, in which symptom-free periods of more than 6 weeks separate recurrences, are distinguished. Frequently resulting from inadequate therapy or corticosteroid-use during the index procedure, subsequent recurrences are usually less severe. A recurrence should be treated according to the same procedures as for the first event. Pericardiectomy may be considered the last resort is severely refractory recurrent pericarditis, but its results are unpredictable. Prognosis of the disease is excellent, as severe complications are rare. | |||
=== Pericardial effusion === | |||
{| class="wikitable" border="0" style='float: left' | |||
|- align='left' | |||
!Table Causes of pericardial effusion<br /> | |||
|- align='left' | |||
| Any type of acute pericarditis | |||
| Cardiac surgery | |||
| Acute myocardial infarction | |||
| Heart failure | |||
| Chronic renal failure | |||
| Iatrogenic | |||
| Metabolic diseases | |||
| Autoimmune diseases | |||
| Trauma | |||
| Chylopericardium | |||
| Pregnancy | |||
| Idiopathic | |||
|} | |||
Fluid accumulation in the pericardium, pericardial effusion, is a common finding on routine echocardiography, and is asymptomatic in the absence of inflammation or cardiac tamponade. It may result from any disease of the pericardium, or be iatrogenic. Most frequently it results from idiopathic pericarditis, malignancy, or iatrogenic defects. | |||
Where the use of electrocardiography and chest radiography is limited in pericardial effusion, echocardiography may reveal an echo-free space in the anterior or posterior sacs, present throughout the cardiac cycle. The absence of cavity collapse indicates the absence of tamponade. | |||
Treatment of pericardial effusion depends on the extent of symptoms, and the etiology underlying the effusion. Asymptomatic mild pericardial effusion (<10mm sum of echo-free spaces in anterior and posterior sacs) may be left untreated. Control echocardiography is indicated at 3-6 months. In moderate (10-20mm sum of echo-free space) to large effusions, a complete history, routine physical, ECG, chest radiography and routine blood analysis is indicated. Treatment is then based upon its expected etiology, standard treatment with aspirin or NSAIDs to relief pain, with invasive procedures indicated in case of tamponade with hemodynamic compromise or recurrent pericarditis as discussed previously. Specific etiologies of pericardial effusion must be managed accordingly. | |||
==== Chronic pericardial effusion ==== | |||
Pericardial effusion is considered chronic when moderate to large effusions persist for at least 3 months. Resulting most frequently from idiopathic cause, intrapericardial pressure is frequently elevated in these patients, which may lead to unexpected tamponade in up to 30% of patients. Hence, pericardiocentesis is indicated to alleviate the fluid accumulation, and pericardiectomy should be considered when large effusions recur. Long term outcome is excellent with this approach. | |||
=== Constrictive pericarditis === | |||
The pericardial layers may become rigid, thickened, and may fuse, resulting in restriction of cardiac filling; constrictive pericarditis. In contrast to cardiac tamponade, where cardiac is hampered throughout diastole, cardiac filling is prohibited in the last two-thirds of diastole in constrictive pericarditis, with preserved abrupt filling in early diastole. | |||
==== Chronic constrictive pericarditis ==== | |||
Any form of pericarditis may end in constrictive pericarditis, presenting with chronic fatigue, dyspnoea, jugular distension, proto-diastolic pericardial knock, hepatomegaly, ascites, peripheral oedema, and pleural effusion. Atrial fibrillation is a common finding, and diffuse flattened or negative T-waves are usually present. These suggestive clinical findings, in addition to a physiology of restriction or constriction on echocardiography, and the presence of a thickened pericardium provide the diagnosis. However, a thickened pericardium may be absent, which does not rule out constrictive pericarditis. Pericardiectomy is the only effective treatment, which should be instituted shortly after diagnosis, as surgical mortality increases with increasing age and functional impairment. | |||
Revision as of 15:46, 17 April 2012
Content is incomplete and may be incorrect. |
Myocardial Disease
Overall, myocardial disease can be subdivided into two types: primary and secondary myocardial disease. Whereas the primary type most commonly has a genetic cause, secondary myocardial diseases are mostly acquired but may be precipitated by a genetic background.
Primary myocardial disease
Five different groups of primary myocardial disease exist; which are defined as ‘diseases of the myocardium with impaired cardiac function’, also referred to as cardiomyopathies.
- Hypertrophic cardiomyopathy (HCM)
- Dilated cardiomyopathy (DCM)
- Restrictive cardiomyopathy (RCM)
- Arrythmic cardiomyopathy (ACM)
- Unclassified cardiomyopathy (UCM)
Hypertrophic cardiomyopathy
The modern description of hypertrophic cardiomyopathy (HCM) dates from 1958. The essential characteristics of HCM are unexplained hypertrophy of the left ventricle in the absence of causative other cardiac or systemic disorders. Distinctive features further comprise myocyte disarray, familial occurrence, and an association with sudden cardiac death (SCD).
Epidemiology
The prevalence of the HCM phenotype was found to be approximately 0.2%, or 1 in 500, in several epidemiological studies. This frequency is notably higher than its occurrence in daily clinical practice. Hence, a large amount of patients remains undiagnosed, most probably without symptoms of inferences for their prognosis.
Genetics
| Table 1. sarcomeric genes associated with HCM |
|---|
|
The identification of the genetic background of HCM resulted in the hypothesis that HCM is a disease of the sarcomere; the contractile unit of the cell. First, mutations were found in the cardiac B-myosin heavy chain gene, while later on other sarcomeric proteins were found to play a role in HCM (Table 1).
It was shown that macroscopic hypertrophy of the myocardium is not essential for neither diagnosis nor prognosis, as mutations for example in troponin T may lead to only minor or no hypertrophy, whereas it is associated with a high incidence of SCD. Nowadays HCM is viewed upon as a genetic disorder, inherited mainly autosomal dominant; with an incomplete and age-related penetrance. Pathological findings then include myocardial hypertrophy, small-vessel disease and myocyte disarray with or without fibrosis.
The prevalence of HCM in the adult population is approximately 1 in 500. But as in only 60% of HCM patients mutations in the abovementioned known sarcomeric genes are present, non-sarcomeric variants of HCM, ‘phenocopies’, have gained interest. Part of these may actually be explained by unidentified defects in sarcomeric genes, but it is unlikely that this adds up to the missing 40%.
The characteristics of phenocopies of HCM differ from sarcomeric HCM; increased incidence of conduction disease and progression to cavity dilation and heart failure. Left ventricular hypertrophy in young children is known not to be caused by sarcomeric HCM, but rather by metabolic disorders and syndromes with equivocal extracardiac features. These diseases may be causal to non-sarcomeric HCM cases. As they include Anderson-Fabry disease [ESC4] and Danon disease, recognition and diagnosis of phenocopies of HCM may alter familial counselling, clarify the presence of extracardiac features, and may even influence treatment.
Pathophysiology
Left ventricular outflow tract obstruction
The subdivision of HCM into obstructive and non-obstructive forms is of clinical relevance, and is based on the presence or absence of a LV outflow tract gradient under resting and/or provocated conditions. These gradients result in a loud apical systolic ejection murmur. The obstruction results in increased intraventricular pressures, impairing LV function by increasing myocardial wall stress and oxygen demand. The obstruction is located either sub-aortic or mid-cavity, where the sub-aortic location is most common, and is caused by systolic anterior motion (SAM) of the mitral valve and mid-systolic contact with the ventricular septum.
SAM is thought to be facilitated by either an abnormal valvular apparatus loose enough to allow movement, or a hemodynamic force with an anterior component during systole. A drag-effect probably attributes to SAM, which refers to the force exerted by a fluid in the direction of the flow. Apart from its role in sub-aortic obstruction, SAM also results in concomitant mitral regurgitation, with the jet directed posteriorly.
The sub-aortic gradient and associated LV pressure increase are pathophysiologic and are prognostically important in patients with HCM. LVOTO is an independent predictor for HCM-related death, progression of the disease in terms of New York Heart Association (NYHA) class III or IV, and death due to heart failure and stroke. A gradient threshold of 30 mmHg is prognostically important, but a further increase is not associated with increased risks. Chronic outflow tract obstruction results in an increase in LV wall stress, myocardial ischemia and fibrosis, and justifies intervention in severely symptomatic patients when optimal medical management is insufficient.
HCM patients can be divided into hemodynamic subgroups based on the representative peak instantaneous gradient as assessed with continuous wave Doppler: 1) obstructive gradient under basal (resting) conditions equal to or greater than 30 mm Hg (2.7 m/s by Doppler), 2) latent (provocable) obstructive—gradient less than 30 mm Hg under basal conditions and equal to or greater than 30 mm Hg with provocation 3) nonobstructive—less than 30 mm Hg under both basal and (provocable) conditions. LV outflow gradients are routinely measured noninvasively with continuous wave Doppler echocardiography. To define latent gradients during and/or immediately following exercise for the purpose of major management decisions, treadmill or bicycle exercise testing in association with Doppler echocardiography is probably the most physiologic and preferred provocative test, given that HCM-related symptoms are typically elicited with exertion. Intravenous administration of dobutamine is undesirable.
Diastolic dysfunction
HCM is, in contrast to other cardiomyopathies, characterized by diastolic dysfunction (both active and passive phases), while systolic function is preserved. The passive relaxation during filling of the ventricle is hampered by an increased chamber stiffness, increasing filling pressures and decreasing myocardial blood flow. Isovolumetric relaxation in early diastole is prolonged in HCM. Diastolic dysfunction may well lie at the basis of heart failure in nonobstructive HCM with preserved systolic function.
Ischemia
Myocardial hypertrophy disrupts the subtle equilibrium of myocardial blood flow. Patients with HCM show an increased baseline flow velocity compared to healthy individuals; reflecting a decreased microvascular resistance to adapt to the increase in oxygen demand. Consequently, coronary flow reserve is decreased. Apart from the changes in the coronary microcirculation, systolic extravascular compression might play a role. Most importantly, HCM results in a progressive mismatch between muscle tissue and vascular growth, resulting in a high risk of myocardial ischemia especially for the subendocardial layers. The presence of myocardial ischemia is an important determinant of progression of the disease as is promotes scarring and remodelling of the ventricle.
Arrhythmia
Myocardial fibrosis associated with HCM is an important arrhythmogenic substrate. Functional consequences of HCM may provide the trigger for ventricular arrhythmias, i.e. ischemia and LVOTO, resulting in non-sustained ventricular tachycardia in approximately 20% of patients. Other functional consequences as diastolic dysfunction, mitral regurgitation, as well as LVOTO are associated with atrial fibrillation (AF) which is observed in 20-25% of HCM patients.
Wall thinning and cavity dilation
Thinning of the LV wall is present frequently in patients with severe LVH and may account for the lack of marked LVH in the elderly. While mechanisms of LV remodelling in HCM are still to be defined, cavity dilation and hampered systolic function occur in less than 5% of patients.
Clinical diagnosis
Echocardiography
Two-dimensional echocardiography is the easiest diagnostic modality for assessment of HCM, but cardiac magnetic resonance imaging (CMR) may be used when echocardiography is inconclusive, or when more detailed anatomic information is needed for clinical decision making.
Echocardiographic characteristics include thickening of the left ventricular wall without cavital dilatation, and a normal or hyperdynamic left ventricular chamber. Although some of its synonyms are misleading, left ventricular outflow tract obstruction is not an invariable characteristic of HCM, and although the cut-off for maximal wall thickness is 15 mm for HCM, some underlying mutations are associated with only minor LVH but a high risk of sudden cardiac death. Systolic-anterior motion of the mitral valve is another typical echocardiographic characteristic of HCM.
Echocardiographic diagnostic criteria for HCM are:
Major:
- LV wall thickness ≥13 mm in the anterior septum or ≥15 mm in the posterior septum or free wall
- Severe SAM (septum–leaflet contact)
Minor:
- LV wall thickness of 12mm in the anterior septum or posterior wall or of 14mm in the posterior septum or free wall
- Moderate SAM (no septum–leaflet contact)
- Redundant mitral valve leaflets
Electrocardiography
Electrocardiographic signs of HCM are typical as the increase in myocardial tissue increases the QRS complexes. Therefore a typical ECG characteristic of HCM is that it meets voltage criteria for LVH and shows changes in repolarization.
Electrocardiographic diagnostic criteria for HCM are:
Major:
- Left ventricular hypertrophy and repolarization changes
- T-wave inversion in leads I and aVL (≥3mm) (with QRS–T wave axis difference ≥30°), V3–V6 (≥3mm) or II and III and aVF (≥5mm)
- Abnormal Q (>40 ms or >25% R wave) in at least two leads from II, III, aVF (in absence of left anterior hemiblock), V1–V4; or I, aVL, V5–V6
Minor:
- Complete bundle branch block or (minor) interventricular conduction defect (in LV leads)
- Minor repolarization changes in LV leads
- Deep S V2 (>25mm)
The diagnosis is confirmed when either 1 major or 2 minor echocardiographic criteria, or 1 minor echocardiographic and 2 minor electrocardiographic criteria are present. Specificity of these criteria relies significantly on the a-priori chance of HCM, and is therefore only relevant in first-degree relatives of index cases with confirmed HCM.
Medical treatment
Asymptomatic patients should only receive drugs when severe LVH is present. Verapamil is the treatment of choice, improving diastolic filling and relaxation of the ventricle, decreasing diastolic filling pressures.
In symptomatic patients, first line medical treatment consists of a calcium-channel blocker. Verapamil is the first choice, but diltiazem may be used as an alternative. Second, beta-blockers may be used when symptoms prevail, and can be used solitarily or in combination with a calcium-channel blocker. In severely symptomatic patients, diuretics may be used with caution, as a small drop in filling pressure may reduce stroke volume and cardiac output dramatically in HCM patients. A combination can be made with either calcium-channel blockers or beta-blockers.
Patients presenting with ventricular tachyarrhythmia or supraventricular atrial fibrillation amiodarone may be used, and may even improve symptoms and prognosis in HCM patients. Disopyramide has negative inotropic action and results in peripheral vasoconstriction and may improve symptoms.
Invasive treatment of obstructive HCM
In patients where maximal medical treatment does not control the symptoms, invasive debulking of the myocardial septum may be considered when a marked outflow gradient is present. Treatment options comprise percutaneous alcohol septal ablation, or surgical septal myectomy.
Surgical myectomy has shown excellent long-term result, but 15-20% of patients may suffer from ventricular remodelling and dilatation of the left ventricle. Since the introduction of alcohol ablation, surgical myectomy is reserved for patients with HCM with concomitant disease that independently warrants surgical correction, such as coronary artery bypass grafting of valve repairs, in whom surgical myectomy can be performed as part of the operation (Guideline AHA 2011).
Septal ablation is considered eligible in patients with outflow tract gradients of more than 30 – 50 mmHg at rest or 60-100 mmHg after provocation. By injection of 1-3 mL of pure alcohol over 5 minutes into the first or second septal branch, a small myocardial infarction is created. Furthermore, the alcohol induces septal hypokinesis, thereby reducing the outflow tract gradient. This gradient may resoilve immediately, or it may take weeks to months. When the outflow tract obstruction persists, patients can be treated a second time.
Dual chamber pacing
In patients with medically refractory symptoms, whom are suboptimal candidates for invasive septal reduction treatment, permanent dual chamber pacing may be considered. Pacing may alleviate symptoms by decreasing the outflow tract pressure gradient. However, maintaining a reduction in gradient requires pre-exitation of the right ventricular apex and distal septum, and complete ventricular caption. For optimal results, this should therefore be performed in highly experienced centers only.
Prognosis and outcome
| Table 2. Risk factors for SCD | |
|---|---|
| Major | Possible |
|
|
In general, symptoms of HCM increase with age. Mortality rates have been reported to account between 2 and 3% per year. Most importantly, patients with HCM may be at high risk of sudden cardiac death, which may even be the disease presentation in particular in asymptomatic or mildy symptomatic young patients. HCM is the most common cause of SCD in young people, including athletes. The pathophysiological basis for this predilection is unclarified, and although SCD is most frequent in young people less than 30 to 35 years old, a risk for SCD extends beyond mid-life. Although HCM presentation and expression is heterogeneous, and its relatively low prevalence, clinical markers as shown in Table 2 may identify patients at high risk for SCD. Patients at an unacceptably high risk of SCD are eligible for ICD implantation.
Dilated cardiomyopathy
Dilated cardiomyopathy (DCM) is a primary myocardial disease characterized by ventricular dilatation (one or both ventricles) and impaired myocardial contractility. The impairment of myocardial function cannot be explained by abnormal loading conditions alone, such as valve disease or systemic hypertension. In at least 50% of patients with DCM, its cause cannot be determined which is referred to as idiopathic DCM. DCM is a condition which causes and presentations are highly variable. The diagnosis of idiopathic DCM should only be made after exclusion of the specific cardiomyopathies with a dilated phenotype.
Epidemiology
The prevalence of DCM is approximately 36 per 100 000.
Genetics
The genetic background of DCM is not as clear as in HCM. Although previously thought to be sporadic, genetic transmission is now thought to account for 30-40% of cases. Multiple genes have been identified that are linked with the occurrence of DCM. Genetic disease may account in part for the primary forms of DCM, but importantly, genetic predisposure may well lead to DCM in case of exposure to precipitating factors such as (emotional) stress, excessive alcohol use or stress upon the cardiovascular system; secondary DCM.
The expression of DCM in the familial form is frequently incomplete, and hence its prevalence has been severely underestimated. Even minor abnormalities may progress into overt DCM, and accurate clinical screening of (asymptomatic) relatives is mandatory for identification of familial DCM cases.
Pathophysiology
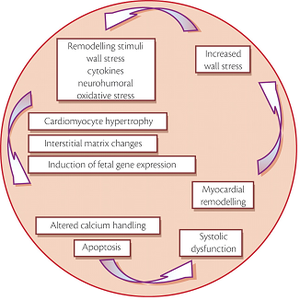
Probably facilitated by a genetic predisposure, DCM can be precipitated by a wide variety of factors including arterial hypertension, myocarditis, or tachyarrhythmias. A subsequent increase in wall stress combined with activation of neurohumoral pathways induces complex cellular and molecular maladaptation. Programmed cell death finally leads to a decrease in the number of functioning cardiomyocytes. This process of cardiac remodelling in itself results in systolic and/or diastolic dysfunction leading to increased wall stress, thereby creating the vicious circle of systolic dysfunction.
The failing myocardium has several distinct factors promoting apoptosis of cardiomyocytes in vitro; cathecholamins, wall stress, angiotensin II, nitric oxide and inflammatory cytokines. Hence, medical management of DCM aims at antagonizing these pathways, reducing stress signalling in and remodelling of the failing heart.
Clinical diagnosis
The most common first manifestation of DCM is heart failure, in which clinical symptoms do not differ from heart failure of other causes. An important feature of the physical examination is a gallop rhythm of S3 and S4, invariably present in DCM. S3 and S4 may fuse in tachycardic patients with new onset of heart failure. Special attention should focus upon excluding valvular heart disease as a cause, and the possibility of right-sided involvement should be considered.
Diagnostic testing in DCM should focus on identification of reversible causes, and includes plasma biomarkers, noninvasive imaging, electrocardiography and exercise testing.
Echocardiography is an important diagnostic modality in DCM, as it can be used to assess both the size and shape of the LV, but also to determine LV function by assessing the LV ejection fraction (EF). Furthermore, valvular heart disease or pericardial abnormalities can be excluded. CMR evaluation may contribute to identification of specific cardiomyopathic conditions, especially when echocardiographic images are suboptimal. Cardiopulmonary exercise testing is a measure of the cardiac response to exertion, and is an established risk factor in DCM patients. Furthermore, the combination of anaerobic threshold and ventilatory efficiency is a reliable predictor for 6-month mortality. Dilatation of the ventricular cavity results in myocyte stretch. In response, B-type natriuretic peptide is released, which is a neurohorme that can be used to evaluate progression of DCM and to guide medical treatment. High plasma concentrations (twice the ULN) are furthermore associated with increased long term mortality.
Electrocardiography does not provide an accurate diagnostic mean in DCM, but can identify several features associated with impaired prognosis, or identify contributing factors to DCM. Where sinus tachycardia is frequently present, non-specific ST-segment or T-wave changes, as well ass changes in P-wave morphology may well be present.
AF is an important feature associated with high mortality; its control may contribute to optimalise cardiac output. Furthermore, the presence of AF may indicate tachycardia-induced cardiomyopathy. 24-hour Holter monitoring can reveal a decreased heart rate variability or complex ventricular arrhythmias which are associated with a high risk for mortality. Finally, prolonged QTc intervals are associated with high mortality.
Management of DCM
Management of symptoms and progression of DCM, coincide with treatment options as described in the management of heart failure. Hence, also in DCM, diuretics and neurohumoral antagonists provide the basis for management of symptoms, and preventive cardio defibrillator or pacemaker implantation is indicated in selected patients with DCM. Most importantly surgical or percutaneous correction of underlying conditions facilitating progression of DCM, such as coronary artery disease, valvular heart disease or congenital abnormalities is warranted.
Specific dilated cardiomyopathies
It is important to note that there are several causes of secondary DCM. A foursome of these are of utmost importance to recognize early on, as accurate diagnosis influences the patients treatment strategy and chance for recovery.
Tako-tsubo (Stress)
Tako-tsubo (octopus pot) cardiomyopathy is an acute cardiomyopathy precipitated by exposure to high doses of cathecholamines. It is most common in middle-aged women, and is usually completely reversible wit supportive care. Diagnostic features include electrocardiographic signs of myocardial infarction, without angiographic evidence of coronary artery disease. A distinctive feature is the presence of apical and midventricular systolic dysfunction in which the base of the heart is relatively spares, which is referred to as apical ballooning. Endomyocardial biopsy may mimic myocardial infarction, and demonstrates contraction band necrosis, but may be useful to exclude myocarditis.
Peripartum cardiomyopathy
Peripartum cardiomyopathy is defined as ‘a cardiomyopathy manifesting between the last month of pregnancy and 6 months post partum’. Most probably, inflammatory factors play a prominent role in its aetiology, but it remains to be fully elucidated. The initial period may feature severe hemodynamic compromise, but if patients survive this period long-term prognosis is excellent, although these women are at higher risk of reccurrence with a subsequent pregnancy.
Peripartum cardiomyopathy
Peripartum cardiomyopathy is defined as ‘a cardiomyopathy manifesting between the last month of pregnancy and 6 months post partum’. Most probably, inflammatory factors play a prominent role in its aetiology, but it remains to be fully elucidated. The initial period may feature severe hemodynamic compromise, but if patients survive this period long-term prognosis is excellent, although these women are at higher risk of reccurrence with a subsequent pregnancy.
Alcoholic cardiomyopathy
Alcoholic cardiomyopathy is a dose-related disorder that resembles idiopathic DCM. Cessation of alcohol use results in an improvement of the disease. Not only does alcohol have a direct toxic effect on the myocardium, excessive alcohol use also increases risk for other comorbidities that increase cardiovascular risk such as systemic hypertension.
Prognosis and outcome
DCM has a highly variable clinical course. Approximately half of DCM patients respond well to contemporary heart failure medication, and an minority of patients show a healing course. Conversely, a subgroup can be identified with a highly unfavourable clinical course, not responsive to heart failure medication and rapidly progressing to inotropy- or LVAD dependency. Overall, 5-year survival rates approximates 30%.
Restrictive and infiltrative cardiomyopathy
Restrictive cardiomyopathy is characterized by an increase in ventricular wall stiffness, impairing its diastolic function. Systolic function is usually preserved in early stages of the disease, but may deteriorate with progression of the disease. RCM is less frequent in the developed world than the previously described HCM and DCM, but is an important cause of death in Africa, India, South and Central America, and Asia due to the high incidence of endomyocardial fibrosis. The spectrum of restrictive cardiomyopathies can be classified as shown in table xx, according to its cause. An important differentiation is that between RCM and constrictive pericarditis. Constrictive pericarditis is similarly characterized by impaired ventricular filling with preserved systolic function, but may be adequately treated by pericardiectomy.
Restrictive (Non-infiltrative)
Idiopathic cardiomyopathy Familial cardiomyopathy
Infiltrative
Amyloidosis
Amyloidosis is a disease that results from tissue deposition of fibrils that have a distinct secondary structure of a beta-pleated sheet configuration, leading to characteristic histological changes. Amyloid depositions can occur in almost any organ, but remains clinically undetected unless extensive depositions are present.
Types of amyloidosis
The most frequent types of amyloidosis are the AL (primary) and AA (secondary) types. AL amyloidosis is a plasma cell dyscrasia, which can occur solitarily or in association with multiple myeloma. AA amyloidosis can be considered a complication of chronic inflammatory disease states such as rheumatoid arthritis, in which the deposits consist of fragments of serum amyloid A, which is an acute phase reactant.
Hereditary amyloidosis has been increasingly recognized in the last decade, and results from mutations in the gene for thransthyretin. Some mutations are clinically limited to the myocardium. Its incidence increases with increasing age, with a predilection for men, but its prognosis is better than that of the AL type. Senile systemic amyloidosis results from deposition of normal wild-type transthyretin. This form of amyloidosis is clinically predominated by an infiltrative cardiomyopathy, but progression is slow and prognosis is better than of other acquired forms.
- Cardiac amyloidosis
- Cardiac amyloidosis is a progressive infiltrative cardiomyopathy. The primary form carries the highest cardiac involvement of approximately one third to half of patients, where deposits may be present even in the absence of clinical symptoms. Secondary amyloidosis is less frequently accompanied by cardiac infiltration, approximately 5% of cases, and is less likely associated with ventricular dysfunction due to a smaller size and more favourable location of the depositions. Familial amyloidosis is associated with clinical signs of cardiac involvement in a quarter of patients, typically presenting after the after of 35 with a distinct involvement of the cardiac conduction system. In senile amyloidosis, extense of deposits may vary widely; from solitarily atrial involvement up to extensive ventricular infiltration.
- Clinical manifestations
- Apart from the occurrence of cardiac disease in the presence of known AL amyloidosis or connective tissue disease or other chronic inflammatory disorder, ardiac amyloidosis should be considered in case of:
- restrictive cardiomyopathy of unknown origin
- Left ventricular hypertrophy with a converse low-voltage ECG
- Congestive heart failure of unknown origin, not responding to contemporary medical management.
- Apart from the occurrence of cardiac disease in the presence of known AL amyloidosis or connective tissue disease or other chronic inflammatory disorder, ardiac amyloidosis should be considered in case of:
- Clinical diagnosis
- Diagnostic testing should include a 12-lead ECG, possibly with Holter monitoring, and routine echocardiography. Specific characteristics are a low-voltage 12-lead ECG with increase septal and posterior wall ventricular thickness.
- Physical examination
- A physical examination may reveal an elevated jugular venous pressure, and signs of systemic edema. Auscultation frequently reveals an apical murmur due to mitral regurgitation, and a third heart sound, but the presence of a fourth heart sound may exclude amyloidosis, as atrial infiltration causes impaired atrial contraction.
- Electrocardiography
- Routine 12-lead ECG shows low voltage in the limb leads, and a pseudoinfarct pattern in approximately 50% of patients. Furthermore, conduction abnormalities occur frequently, as does atrial fibrillation.
- Echocardiography
- Thickening of the left ventricular wall with diastolic dysfunction are early echocardiografic features of the disease. In advancing disease, wall thickening increases resulting in a restrictive cardiomyopathy. “Sparkling” myocardium is a distinct characteristic of cardiac amyloidosis, referring to an increased echogenicity of the myocardium. However, only a minority of patients has this pattern. Doppler evaluation shows a restrictive pattern with E dominance and a short deceleration time.
- Furthermore, intracardiac thrombus is frequently present, which is associated with atrial fibrillation and left ventricular diastolic dysfunction.
- The thickening of the ventricular wall caused by amyloidosis may be misinterpreted as hypertrophy on echocardiography. An important distinctive characteristic of amyloidosis is the voltage-to-mass ratio. Unlike normal hypertrophic myocardium, the increased ventricular mass in amyloidosis is associated with a decrease in electrocardiographic voltage.
- Management
- Few treatments for cardiac amyloidosis exist, and available treatments are dependent on the type of amyloidosis present. Hence, typing of the disease is pertinent. AL amyloidosis may be treated with chemotherapy using alkylating agents alone or in combination with bone marrow transplantation. Heart transplantation in combination with bone marrow transplantation after high-dose chemotherapy was shown to be result in approximately a third of treated patients surviving over 5 years, but as the great majority of patients with AL amyloidosis has severe non-cardiac amyloidosis most of patienst are not suitable transplant candidates. Patients with other types of amyloidosis frequently have less affected hearts, and progression of the disease is slow. AA amyloidosis may respond to anti-inflammatory and imunosupressive drugs that reduce production of the acute-phase reactant protein. If heart failure is present, it is usually more prone to routine medical options to reduce symptoms. If needed, heart transplantation can be performed successfully. In patients where transthyretine is the amyloidogenic protein, liver transplantation may be curative as tranthyretine is produced in the liver, but the cardiac disease may progress regardless in some patients.
- Overall, caution should be taken in prescribing digitalis, nifedipine, verapamil and ACE-inhibitors to cardiac amyloidosis patients. There is a high susceptibility to digitalis intoxication, nifedipine-induced hemodynamic deterioration, verapamil-induced left ventricular dysfunction, and ACE-inhibitor induced profound hypotension.
- If atrial fibrillation is present, or systolic ventricular function is severely impaired anticoagulation is indicated to prevent intracardiac thrombi.
- In selected patients with conduction disorders, pacemaker implantation may be considered. If ventricular function is severely impaired, ICD implantation may be considered.
- Prognosis and outcome
- Prognosis of especially AL amyloidosis is poor.
- Sarcoidosis
- Sarcoidosis is a multisystem inflammatory conditions caharacterized by the formation of non-caseating granulomas, most frequently affecting the lungs and lymphatic system whereas myocardial involvement is seen in one quarter of cases only. Genetic factors are suggested as there was found to be aggregation of cases within families.
- Pathophysiology
- Non-caseating granulomas may infiltrate the myocardium, leading to fibrotic scarring of the myocardium. Involvement of the myocardium may be patchy, resulting in a relatively high likelihood of false-positive results from biopsy. Cardiac sarcoidosis must me differentiated from chronic active myocarditis and giant cell myocarditis.
- Clinical diagnosis
- Patients may present with syncope, heart block or congestive heart failure. Sudden cardiac death may well be a devastating initial manifestation of the disease due to malignant ventricular arrhythmias, but both atrial and ventricular presentations are common. Symptoms of heart failure may result from direct myocardial involvement, but can also be due to extensive pulmonary fibrosis; cor pulmonale. Physical examination may include signs of extracardiac sarcoidosis, a right sided third heart sound, and both S3 and S4, as well as murmurs of tricuspid regurgitation or mitral regurgitation.
- Initial consideration of the diagnosis is often based on chest radiographs showing bilateral hilar lymfadenopathy. CMR is emerging as a highly sensitive and specific test for sarcoidosis, and nuclear imaging techniques may show regional perfusion defect due to the granulomatous inflammation on SPECT, or focal uptake on PET CT. electrocardiography is useful to assess the extent of conduction system involvement. Echocardiography shows left ventricular dilatation with hypokinesis, right ventricular enlargement and hypertrophy and possibly left ventricular aneurysm formation.
- Minimal evaluation of a patients suspected of cardiac sarcoidosis consists of a 12-lead ECG, Holter monitoring and echocardiography.
- Management
- Early detetction of the disease is critical for its clinical course. Immunosuppression using corticosteroids to halt the progression of inflammation is the treatment of choice in sarcoidosis, to which myocardial dysfunction, conduction disturbances and arrhthmias may all respond. Most important is the differentiation of sarcoidosis from giant cell myocarditis, which is a more aggressive disorder requiring intensive medical and mechanical support and frequently necessitates heart transplantation. Pacemaker or ICD implantation is indicated in patients with conduction disorders or malignant arrhythmias, as medical treatment is usually ineffective.
Storage diseases
Hemochromatosis
Hemochromatosis is defined as a disorder of the iron metabolism, resulting in accumulation of iron in parenchymal tissues. In particular cardiac, liver, gonadal and pancreatic involvements are typical for hemochromatosis, in which the toxicity of redox-active iron results in organ dysfunction. Typically, hemochromatosis therefore leads to a combination of heart failure, cirrhosis, impotence, diabetes and arthritis. Although several organ systems are usually involved, cardiac complications predominate as its presenting features, which are dependent on the site and amount of cardiac depositions. DCM is the typical phenotype of cardiac involvement, but a restrictive pattern may be present.
The most frequent, adult-onset, form of hemochromatosis was found arise from mutations in the HFE-gene, coding for a transmembrane protein involved in iron uptake in the liver and the intestine. Less frequently, mutations in the transferrine 2-encoding gene may result in hemochromatosis. The juvenile form of hemochromatosis results from mutations in the genes encoding hepcidin and hemojuvelin. The disease may also be acquired and result from ineffective erythropoiesis secondary to a defect in haemoglobin synthesis, chronic liver disease, chronic excessive oral or parenteral intake of iron, or from multiple blood transfusions.
Three stages of the natural history of adult-onset hemochromatosis can be differentiated. The first stage, the biochemical stage, is characterized by an iron overload which remains confined to the plasma compartment. Transferrin saturation is increased. The second phase, the deposition phase, is characterized by iron accumulation in parenchymal tissues, accommodated by an increase in serum ferritine levels. The third and final stage is that of organ dysfunction.
Juvenile hemochromatosis is a more rapidly progressing disease, which leads to early organ dysfunction, around the age of 30, and in frequently characterized by premature death due to severe cardiac complications.
The invariably present symptoms of heart failure, may frequently be accompanied by arrhythmias, especially ventricular extrasystoles, supraventricular tachycardia, and atrial fibrillation or flutter; either due to atrial iron depositions, or ventricular dysfunction resulting in increased ventricular pressure. Furthermore conduction system involvement may lead to AV block or sick sinus syndrome.
- Diagnostic features
- Symptoms at initial presentation may vary. Echocardiography may show increased left ventricular wall thickness, ventricular dilatation, and ventricular dysfunction. CMR imaging represents a sensitive mean and may aid in early detection of the disease. Electrocardiographic characteristics include ST-segment and T wave abnormalities as well as supraventricular arrhythmias, but occur as the disease advances. Biochemical testing reveals increased elevated transferrine saturation, increase plasma iron levels with low or normal iron binding capacity.
- Management
- Repeated phlebotomy is the cornerstone of hemochromatosis treatment, although chelating agents such as deferoxamine may be considered. Early detection of the disease is critical, as depletion of iron overload may result in complete reversal of symptoms at this stage. Evidence was found that a threshold exists beyond which permanent damage is pertinent, at which stage iron depletion does not result in recovery of function. At end-stage disease, heart transplantation is a viable option with good survival rates. Importantly, screening of first degree relatives is pertinent to ensure early detection of hereditary forms of hemochromatosis.
Fabry disease (angiokeratoma corporis diffusum universale)
Fabry disease is an inheritable deficiency of the lysosomal alpha-galactosidase A, resulting in an accumulation of glycosphingolipids in the lysosomes. There is a wide variety of know mutations, which all result in a different level of alpha-galactosidase inactivity, and hence, clinical manifestation may range from isolated myocardial disease to systemic involvement. Patients may suffer from angina pectoris and myocardial infarction due to the accumulation of the aforementioned lipids in the endothelium of the coronary arteries, but the epicardial vessels show no abnormalities on angiography. Ventricular function is hampere due to thickening of the ventricular walls, which results in impaired diastolic compliance, with a preserved systolic function, which may even precede myocardial hypertrophy. Other common features of the disorder include systemic hypertension, congestive heart failure, and mitral valve prolapse.
The surface electrocardiogram may reveal a short PR interval, atrioventricular block, and ST-segment and T wave abnormalities. Echocardiography may be inconclusive, but CMR imaging may differentiat Fabry disease from other infiltrative processes. Definite diagnosis is made on endomyocardial biopsy.
Treatment of Fabry is safe and effective, and consist of enzyme-replacement therapy.
Gaucher Disease
Gaucher disease is an inheritable deficiency of beta-glucosidase, resulting in accumulation of cerebrosides. Cardiac involvement results in impaired cardiac function due to reduced chamber compliance.
Treatment of Gaucher disease consists of enzyme replacement therapy, or hepatic transplantation as a last resort. Response to treatment with respect to recovery of symptoms may vary.
Glycogen storage disease
Glycogen storage disease may result in cardiac involvement in case of type II, III, IV and V, but survival until adulthood is rare except for type III disease. Cardiac involvement is characterized by left ventricular hypertrophy, electrographically and echocardiographically, but symptoms are frequently absent.
Endomyocardial
Endomyocardial fibrosis
Endomyocardial fibrosis is an important cause of congestive heart failure in equatorial Africa. Studies have shown prevalence up to 20% of the population, mostly familial in children or young adults, and symptoms occurred in a minority of detected cases. The disease predominantly occurs in black individuals, but may rarely present in white subjects. Fibrous lesions hamper cardiac function by impairing the inflow of the ventricles, affecting the left or both ventricles most frequently. Solitaire right-sided involvement is less frequent occurring in approximately 10% of cases. Symptoms are concordant with the ventricles involved, an atrial fibrillation and ascites are known factors associated with a poor prognosis. Clinical diagnosis is based upon clinical presentation, lab testing, and angiography. Left-sided myocardial biopsy is relatively contraindicated, as it may result in systemic emboli.
Endomyocardial fibrosis is a relentless disease, half of patients not making it beyond 2 years after detection, depending on the extent of symptoms at presentation. Medical management is only effective in the early stages of the disease, and is aimed at relief of symptoms. Surgical correction of the results of the disease, i.e. valve replacement, improves hemodynamic characteristics, but mortality is high. Fibrosis may reoccur, but long-term survival can be good.
Hypereosinophilic syndrome: Löffler Endocarditis
The hypereosinophilic syndrome is a systemic disease, involving several organ systems. Cardiac involvement, Löffler endocarditis, is usually present when eosinophil counts are high for a longer period of time. The eosinophilia itself may occur from several different causes.
Histopathology shows eosinophilic myocarditis extending into the subendocardium, endocardial thickening and inflammation of the small intramural coronary vessels.
Clinical features of the disease are weight loss, cough, fever, and a rash. Cardiac involvement may retain asymptomatic in the early stages of the disease, but as the disease progresses as much as 50% of patients develop cardiomegaly or congestive heart failure.
Clinical features of the disease are weight loss, cough, fever, and a rash. Cardiac involvement may retain asymptomatic in the early stages of the disease, but as the disease progresses as much as 50% of patients develop cardiomegaly or congestive heart failure.
Routine care applies to these patients. Diuretics, and neurohumoral blockade are appropriate, as is anticoagulation. Corticosteroids and cytotoxic drugs increase survival in patients with Löffler endocarditis, and interferon may be used as a last option in refractory patients. Surgical therapy may be considered as palliative treatment in the fibrotic fase of the disease.
Arrythmic cardiomyopathy (See ARVC/D)
Unclassified Cardiomyopathy
Left ventricular non-compaction
Secondary cardiomyopathies
Nine different subgroups of secondary myocardial disease exist, which are defined as diseases of the myocardium with a known cause.
Secondary myocardial disease
Myocardial disease with a known origin is termed secondary myocardial disease. Timely correction of the originating disease may result in reversal of the cardiomyopathy. Nine different etiologies can be distinguished:
- Hypertension
- Ischaemia
- Valvular disease
- Alcohol
- Metabolic cardiomyopathy
- Takotsubo cardiomyopathy
- Peripartum cardiomyopathy
- Tachycardia
- Muscular dystrophy
Hypertension or valvular disease
Inadequately treated hypertension or aortic stenosis results in adaptation of the left ventricle by means of hypertrophy. Although primarily considered an adaptive process to systolic overload, hypertrophy of the left ventricle is associated with ventricular dysfunction, arrhythmias, and sudden cardiac death. The process of hypertrophy involves enlargement and proliferation of myocytes, and interstitial fibrosis characterized by deposition of collagen type I and III. With increasing fibrosis, the compliance of the ventricle decreases resulting in loss of diastolic function before systolic function is impaired. Within this process of increasing myocardial mass, the coronary vasculature fails to adapt accordingly. To compensate, the auto-regulatory mechanisms exhaust part of the coronary reserve by decreasing vascular resistance during resting conditions by vasodilation, to accommodate to the increase in myocardial oxygen demand. This renders the myocardium at high risk for ischemia, and hence, patients may suffer from anginal complaints even in the absence of significant coronary artery disease. In patients with LVH, atrial fibrillation and ventricular arrhythmias, including multifocal ventricular extrasystoles, and short runs of ventricular tachycardia, are frequently found. The combination of myocardial fibrosis, maladaptation of the vasculature causing ischemia, autonomic imbalance and a prolongation of the action potential may serve as arrhythmogenic substrate in patients with LVH, resulting in an increased risk of sudden cardiac death.
Adequate antihypertensive treatment, with ACE inhibitors and angiotensine receptor antagonist shown to be most potent, results in a regression of left ventricular mass. Calcium antagonists were also shown to result in LV mass regression, but importantly atenolol has been linked with a controversial increase in cardiovascular mortality in patients with LVH. In patients with aortic stenosis, infinite treatment is valvular replacement to relief the systolic overload of the ventricle.
With regression of the ventricle, improved diastolic, and preserved systolic function result, as well as a relief of vascular maladaptation-induced ischemia. The combination of which results in a decrease in cardiovascular events.
Ischemic cardiomyopathy
Alcohol
| Direct Toxic Effects |
|---|
|
| Toxic Effect of Metabolites |
|
| Nutritional or Trace Metal Deficiencies |
|
| Electrolyte Disturbances |
|
Long-term alcohol abuse, >80g of alcohol per day (equivalent to 1 liter of wine) for more than 5 years, may lead to a dilated form of cardiomyopathy. Alcohol-induced dilated cardiomyopathy is the leading cause of non-ischemic dilated cardiomyopathy, accounting for approximately 50% of cases. Most probably genetic predisposition for DCM plays an important role, as excess alcohol consumptions prevails far more often than alcoholic cardiomyopathy (ACM). Both the direct toxic effects of ethanol and its metabolites, as well as frequently occurring concomitant deficiencies of vitamins, minerals or electrolytes may adversely affect myocardial function (TABLE).
Two stages of ACM are recognized when untreated. The first stage comprises asymptomatical ventricular dilatation in which diastolic dysfunction may be present, at least partly due to interstitial fibrosis of the myocardium. Fifty percent of asymptomatic patients have echocardiographic signs of LVH with preserved systolic function. The second stage is characterized by impairment of systolic function, and clinically overt heart failure. The prognosis of untreated ACM is comparable to DCM, but is far more favourable in patients that abstain from alcohol use, or dramatically reduce alcohol intake (to less than 60g of ethanol per day). Most of the improvement follows abstinence within 6 months, but ventricular function may improve for up to 2 years. Heart failure therapy may improve ventricular function, but has only been shown to benefit survival in patients that practise abstinence.
Metabolic cardiomyopathy
The group of metabolic cardiomyopathies comprises a heterogeneous group of myocardial disease secondary to a disruption in metabolism. Metabolic cardiomyopathy associated with diabetes mellitus is most common. Independent of its influence on hypertension or coronary artery disease, high levels of plasma glucose are increasingly associated with a direct deteriorative effect on ventricular function. Other examples consist of nutritional deficits such as thiamine deficiency, or storage diseases, and mutations in AMP kinase.
Takotsubo cardiomyopathy
The prevalence of takotsubo cardiomyopathy is largely unknown, but the syndrome predominantly affects women between 60 and 65 years of age. Patients with Takotsubo cardiomyopathy present with electrocardiographic features mimicking an acute coronary syndrome in association with elevated cardiac biomarkers, but in the absence of significant coronary artery disease. The disease has inherited its name from the distinct angiographic feature of apical ballooning, resembling an octopus-pot or Tako-tsubo. Left ventricular function is typically impaired in the apical and mid ventricular regions, with preserved basal function, although reverse patterns may be seen. High levels of catecholamine have been suggested to play an important role in the etiology of the syndrome, which can be associated with emotional or physical stress, or in extremes in case of subarachnoidal haemorrhage. This catecholamine storm may induce severe peripheral coronary spasm, leading to its clinical presentation. Treatment usually consists of aspirin, ACE-inhibitors or angiotensin receptor antagonists in case of preserved blood pressure, beta-blockers to reduce heart rate, and nitrates to counteract coronary spasms. LV function may restore rapidly within a few hours or days, even when admission ejection fraction was severely impaired, and clinical outcome is good although the disease may recur in 5% of patients.
Peripartum cardiomyopathy
Left ventricular systolic function impairment within 1 month of delivery, or during the first 5 months post partum, in the absence of pre-existing cardiac disease, and in the absence of another recognized cause for the cardiac dysfunction is termed peripartum cardiomyopathy.
Presentation is typically with features of left ventricular failure, although many features are undistinguishable from normal changes in pregnancy, due to which mild forms may not even be recognized. An inflammatory component has been suggested, in addition to malnutrition, viral infection, an abnormal immune response, and familial predisposition. Recurrence of the disease in subsequent pregnancies is noted, and makes the previously mentioned etiologies hard to explain. Most probably, peripartum cardiomyopathy results from predisposition to DCM, triggered to uncover by the high cardiovascular burden of the pregnancy.
Standard heart failure therapy can be instituted in peripartum cardiomyopathy, but the use of ACE-inhibitors and angiotensin receptor antagonists is contraindicated during pregnancy after the first trimester due to their possible adverse effects on the fetus. In extreme cases, the potential risks for the fetus should however be balanced against the critical need for preservation of ventricular function in order to provide both the mother and the fetus the best chance for a favourable clinical outcome. Pregnancy is also associated with a high risk of thrombo-embolism, as it is a hypercoagulable state per definition, which is enhanced by the presence of an impaired ventricular function in case of peripartum cardiomyopathy, and prophylaxis is therefore recommended.
Despite optimal treatment, LV function may normalize in as less as 50% of patients, and may deteriorate to end-stage heart failure in 15%. Potential recurrence in future pregnancies requires counselling of patients to prevent subsequent episodes of symptomatic heart failure and progression of ventricular dysfunction.
Tachycardia-induced cardiomyopathy
Persistently high heart rates, such as in sustained ventricular tachycardia, or associated with atrial fibrillation, results in heart failure when left untreated. Normalization of the heart rate by means of beta-blockade subsequently leads to normalization of ventricular function, and is therefore the cornerstone in treatment of tachycardia-induced cardiomyopathy.
Cardiomyopathy in muscular dystroph
Defined as primary disorders of skeletal and/or cardiac muscles of genetic etiology, muscular dystrophies were primarily described based upon the distribution and extent of skeletal muscle involvement. The involvement of the heart was commonly attributed to processes extrinsic to the hearts, resulting in restrictive lung disease, subsequent pulmonary hypertension, and secondary myocardial dysfunction. Intrinsic dysfunction is increasingly recognized as an important etiology for myocardial function impairment in the presence of muscular dystrophy. Typical forms of dystrophy are based on deficiency of dystrophin, of which mutations have been described in X-linked DCM. Furthermore, histological changes were found in the myocardium similar to those in skeletal muscles, which suggest a common etiology, and cardiac manifestations may be present even in the absence of myopathic symptoms.
Treatment of cardiac dysfunction is treated according to the nature of cardiac involvement. Conduction disorders may present which require pacing, and standard heart failure therapy may be instituted in case of ventricular dilatation and functional impairment. Ventricular tachyarrhythmia may be found in particular in myotonic dystrophia, and require the implantation of an internal cardiac defibrillator to prevent its associated sudden cardiac death.
Pericardial Disease
The pericardium comprises two layers; the visceral layer that adheres to epicardial surface of the heart, and the parietal layer that surrounds most of the heart. Pericardial disease is common, and diagnosis is usually straightforward as the pericardium reacts to disruption by a wide variety of agents and processes in a relatively uniform manner. Typical presentation is with chest pain and fever, production of pericardial fluid with possible cardiac tamponade, or a constrictive pattern by thickening, retraction and calcification.
Acute pericardial syndromes
Cardiac Tamponade
An increase in intrapericardial pressure, resulting in compression of the heart, and thereby a restriction of cardiac inflow, is termed cardiac tamponade. Tamponade may result from pericardial effusion of any cause. The intrapericardial pressure importantly determines to what extent cardiac inflow is decreased, but two factors need to be taken into consideration. First, intrapericardial pressure is determined not only on the amount of fluid that accumulates, but also on the rate with which this accumulation proceeds, and the available distensibility of the pericardium. Chronic effusions may therefore lead to small increases in intrapericardial pressures in the presence of large fluid accumulations, and small accumulations may directly lead to sever cardiac tamponade for example in free wall rupture. Second, the intravascular volume and intra-atrial, and -ventricular pressures determine at what pressure inflow becomes impaired. When intrapericardial pressure exceeds right atrial pressure (approximately 8 mmHg), tamponade usually follows. However, in patients in whom intra-atrial pressure is decreased, for example due to volume depletion, tamponade may occur at low intrapericardial pressures; low-pressure cardiac tamponade.
With increasing intrapericardial pressure, clinical features increase with severe hemodynamic compromise as its end stage. First, small changes in intrapericardial pressure induce subtle changes in arterial pressure, cardiac output, and variations in arterial pressure with inspiration (pulsus paradoxus). When intrapericardial pressure reaches levels similar to right atrial and diastolic right ventricular pressure echocardiographic evidence of tamponade may be found in diastolic collapsing of the right atrium, and increased variations of blood flow velocity over the cardiac valves with respiration. Fulminant clinical tamponade presents with symptoms that depend on its etiology. Acute cardiac tamponade based upon aortic of free wall rupture presents with syncope and sudden collapse, whereas tamponade in the setting of an acute inflammatory pericardits may present with pericardial chest pain, and dyspnoea. Therefore, in patients presenting with chest discomfort, dyspnoea, tachycardia or tachypnoea cardiac tamponade should be suspected when jugular distension, hypotension or pulsus paradoxus is present. Auscultation may reveal pericardial friction rub, and heart sounds may be faint. Echocardiography shows pericardial effusion, the previously mention diastolic collapsing of the cardiac cavities, increased flow velocity over tricuspid and pulmonary vales, and decreased flow velocity over the aortic and mitral valves.
When secondary to inflammatory pericarditis, up to moderate tamponade may be treated by anti-inflammatory drugs. In severe tamponade, pericardiocentesis should be performed to immediately alleviate intrapericardial pressure. Surgical drainage should be considered when pericardiocentesis is unsuccessful, or when tamponade recurs.
Acute pericarditis
| Table Causes of acute pericarditis |
|---|
| Acute idiopathic pericarditis |
Infectious pericarditis:
|
| Postpericardiotomy syndrome |
| Postmyocardial infarction pericarditis |
| Renal insufficiency |
| Neoplastic disease |
| Chest trauma |
| Irradiation |
| Collagen diseases |
Acute inflammation of the pericardium may result from a wide variety of etiologies TABLE, and typically presents with chest pain, a pericardial friction rub on auscultation, and repolarization changes on the electrocardiogram.
Patients present with a rapid-onset chest pain syndrome, located precordial and retrosternal, and radiating to the subclavian region, the back and the trapezoid region. Chest pain is of moderate severity, lasting for several days, and increases with inspiration or chest movement. Patients typically alleviate the pain by sitting, and leaning forward.
A pericardial friction rub is pathognomonic of pericarditis, and ECG changes are frequently present, which comprise diffuse concave ST-segment elevation, with positive T-waves in several leads. Atrial injury is accompanied by PR-segment depression. After several hours to a few days, ST-segments return iso-electric, and negative T-waves may occurs subsequently and may persist for several weeks, although they frequently normalize within days. Pericardial effusion may be present, and is diagnosed by chest X-ray when fluid accumulation exceeds 250mL, or by echocardiography.
Initial presentation may therefore mimick ST-segment elevation myocardial infarction. Onset of chest is, however, less abrupt in acute pericarditis, and varies with respiration. Diffuse ST-segment changes are present in pericarditis, whereas STEMI presents with ST-segment elevation, and reciprocal depression, in leads corresponding to the ischemic myocardium. Biomarkers may, however, be positive in both syndromes.
Treatment consists of aspirin while pain and fever are present, which usually adequately alleviates symptoms. Another option is NSAIDs, which are recommended when aspirin in insufficient or contraindicated. Corticosteroids should, however, be avoided as they are associated with relapsing pericarditis. Hospital admission may be necessary in patients with high fever, large effusions or cardiac tamponade.
Recurrent pericarditis
In 8 up to 80% of patients, pericarditis recurs after a first episode of acute pericarditis. A continuous type, in which symptoms recur shortly after cessation of anti-inflammatory therapy, and an intermittent type, in which symptom-free periods of more than 6 weeks separate recurrences, are distinguished. Frequently resulting from inadequate therapy or corticosteroid-use during the index procedure, subsequent recurrences are usually less severe. A recurrence should be treated according to the same procedures as for the first event. Pericardiectomy may be considered the last resort is severely refractory recurrent pericarditis, but its results are unpredictable. Prognosis of the disease is excellent, as severe complications are rare.
Pericardial effusion
| Table Causes of pericardial effusion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any type of acute pericarditis | Cardiac surgery | Acute myocardial infarction | Heart failure | Chronic renal failure | Iatrogenic | Metabolic diseases | Autoimmune diseases | Trauma | Chylopericardium | Pregnancy | Idiopathic |
Fluid accumulation in the pericardium, pericardial effusion, is a common finding on routine echocardiography, and is asymptomatic in the absence of inflammation or cardiac tamponade. It may result from any disease of the pericardium, or be iatrogenic. Most frequently it results from idiopathic pericarditis, malignancy, or iatrogenic defects.
Where the use of electrocardiography and chest radiography is limited in pericardial effusion, echocardiography may reveal an echo-free space in the anterior or posterior sacs, present throughout the cardiac cycle. The absence of cavity collapse indicates the absence of tamponade.
Treatment of pericardial effusion depends on the extent of symptoms, and the etiology underlying the effusion. Asymptomatic mild pericardial effusion (<10mm sum of echo-free spaces in anterior and posterior sacs) may be left untreated. Control echocardiography is indicated at 3-6 months. In moderate (10-20mm sum of echo-free space) to large effusions, a complete history, routine physical, ECG, chest radiography and routine blood analysis is indicated. Treatment is then based upon its expected etiology, standard treatment with aspirin or NSAIDs to relief pain, with invasive procedures indicated in case of tamponade with hemodynamic compromise or recurrent pericarditis as discussed previously. Specific etiologies of pericardial effusion must be managed accordingly.
Chronic pericardial effusion
Pericardial effusion is considered chronic when moderate to large effusions persist for at least 3 months. Resulting most frequently from idiopathic cause, intrapericardial pressure is frequently elevated in these patients, which may lead to unexpected tamponade in up to 30% of patients. Hence, pericardiocentesis is indicated to alleviate the fluid accumulation, and pericardiectomy should be considered when large effusions recur. Long term outcome is excellent with this approach.
Constrictive pericarditis
The pericardial layers may become rigid, thickened, and may fuse, resulting in restriction of cardiac filling; constrictive pericarditis. In contrast to cardiac tamponade, where cardiac is hampered throughout diastole, cardiac filling is prohibited in the last two-thirds of diastole in constrictive pericarditis, with preserved abrupt filling in early diastole.
Chronic constrictive pericarditis
Any form of pericarditis may end in constrictive pericarditis, presenting with chronic fatigue, dyspnoea, jugular distension, proto-diastolic pericardial knock, hepatomegaly, ascites, peripheral oedema, and pleural effusion. Atrial fibrillation is a common finding, and diffuse flattened or negative T-waves are usually present. These suggestive clinical findings, in addition to a physiology of restriction or constriction on echocardiography, and the presence of a thickened pericardium provide the diagnosis. However, a thickened pericardium may be absent, which does not rule out constrictive pericarditis. Pericardiectomy is the only effective treatment, which should be instituted shortly after diagnosis, as surgical mortality increases with increasing age and functional impairment.