CPVT: Difference between revisions
mNo edit summary |
|||
| Line 29: | Line 29: | ||
[[File:Ventricular_complexes.png|thumb|300px|right]] | [[File:Ventricular_complexes.png|thumb|300px|right]] | ||
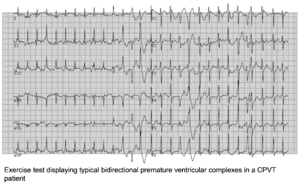
The resting ECG in CPVT is normal. However, there is a progressive ventricular ectopy as heart rate increases during exercise or isoproterenol infusion. Both the frequency and the complexity of the ventricular ectopy increase with the work load. It generally starts with monomorphic premature ventricular complexes (PVCs) and is followed bigemini and subsequently more complex arrhythmias, including doublets triplets, bidirectional ventricular tachycardia to polymorphic ventricular tachycardia. Most of the times, the arrhythmia is self-limiting. However, in some patients, especially if exercise is continued, repeated polymorphic VTs can deteriorate in ventricular fibrillation and sudden cardiac death. | The resting ECG in CPVT is normal. However, there is a progressive ventricular ectopy as heart rate increases during exercise or isoproterenol infusion. Both the frequency and the complexity of the ventricular ectopy increase with the work load. It generally starts with monomorphic premature ventricular complexes (PVCs) and is followed bigemini and subsequently more complex arrhythmias, including doublets triplets, bidirectional ventricular tachycardia to polymorphic ventricular tachycardia. Most of the times, the arrhythmia is self-limiting. However, in some patients, especially if exercise is continued, repeated polymorphic VTs can deteriorate in ventricular fibrillation and sudden cardiac death. | ||
| Line 36: | Line 38: | ||
[[File:CPVT.jpg|thumb|200px|left]] | [[File:CPVT.jpg|thumb|200px|left]] | ||
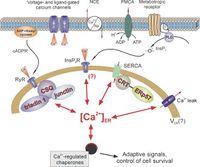
CPVT is caused by mutations in genes involved in the calcium homeostasis of cardiac cells. Four disease-causing genes have been identified: the ryanodine<cite>ryanodine</cite> receptor 2 gene (RyR2) (60%), the cardiac calsequestrin 2 gene (CASQ2) (1-2%), the calmodulin gene (CALM1) (<1%) and the TRDM gene (<1%). Mutant RyR2 channels have a gain-of-function effect, resulting in excessive calcium release during sympathetic activation. Mutant CASQ2 causes loss of buffering capacity for calcium of the sarcoplasmatic reticulum. The mutations cause excessive calcium in the myocyte cytosol generating depolarizing membrane currents, which in turn lead to delayed afterdepolarizations and cardiac arrhythmias. | CPVT is caused by mutations in genes involved in the calcium homeostasis of cardiac cells. Four disease-causing genes have been identified: the ryanodine<cite>ryanodine</cite> receptor 2 gene (RyR2) (60%), the cardiac calsequestrin 2 gene (CASQ2) (1-2%), the calmodulin gene (CALM1) (<1%) and the TRDM gene (<1%). Mutant RyR2 channels have a gain-of-function effect, resulting in excessive calcium release during sympathetic activation. Mutant CASQ2 causes loss of buffering capacity for calcium of the sarcoplasmatic reticulum. The mutations cause excessive calcium in the myocyte cytosol generating depolarizing membrane currents, which in turn lead to delayed afterdepolarizations and cardiac arrhythmias. | ||
Revision as of 05:30, 22 March 2013
Auteur: Louise R.A. Olde Nordkamp
Supervisor: Arthur A.M. Wilde
Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) refers to a hereditary disease that is associated with exercise (or adrenergic) induced ventricular arrhythmias and/or cardiac syncope and carries an increased risk of sudden cardiac death.
General features
- The diagnosis is based on adrenergic-induced bidirectional and polymorphic ventricular tachycardia.
- The resting ECG is normal and the heart is structurally normal.
- It is an inheritable cardiac arrhythmia syndrome[1] with an autosomal dominant (RyR2) or recessive (CASQ2) inheritance.
- The arrhythmias typically occur in children[2] and adolescents.
- The mortality rate is approximately 31% by the age of 30 years, if untreated.
- The prevalence is estimated to be 1:10.000 in Europe.
Clinical diagnosis
The clinical diagnosis of CPVT is confirmed in an individual with polymorphic VT reproducibly induced during exercise tests, isoproterenol infusion or emotion and exercise.
Patients present themselves most often with syncope or even an out-of-hospital cardiac arrest, usually during the first or second decade of life. The symptoms are always triggered by exercise or emotional stress. A family history of exercise-related syncope, seizure or sudden death is reported in 30% of the patients.
Family members of patients with CPVT who also carry a mutation in a CPVT-associated gene are often asymptomatic.
Physical examination
Patients can present with symptoms of arrhythmias during exercise:
- Out-of-hospital-cardiac-arrest
- Syncope, pre-syncope (weakness, lightheadedness, dizziness)
ECG tests
The resting ECG in CPVT is normal. However, there is a progressive ventricular ectopy as heart rate increases during exercise or isoproterenol infusion. Both the frequency and the complexity of the ventricular ectopy increase with the work load. It generally starts with monomorphic premature ventricular complexes (PVCs) and is followed bigemini and subsequently more complex arrhythmias, including doublets triplets, bidirectional ventricular tachycardia to polymorphic ventricular tachycardia. Most of the times, the arrhythmia is self-limiting. However, in some patients, especially if exercise is continued, repeated polymorphic VTs can deteriorate in ventricular fibrillation and sudden cardiac death.
Genetic diagnosis
CPVT is caused by mutations in genes involved in the calcium homeostasis of cardiac cells. Four disease-causing genes have been identified: the ryanodine[3] receptor 2 gene (RyR2) (60%), the cardiac calsequestrin 2 gene (CASQ2) (1-2%), the calmodulin gene (CALM1) (<1%) and the TRDM gene (<1%). Mutant RyR2 channels have a gain-of-function effect, resulting in excessive calcium release during sympathetic activation. Mutant CASQ2 causes loss of buffering capacity for calcium of the sarcoplasmatic reticulum. The mutations cause excessive calcium in the myocyte cytosol generating depolarizing membrane currents, which in turn lead to delayed afterdepolarizations and cardiac arrhythmias.
Risk Stratification and Treatment
Risk stratification and treatment is best provided by an expert cardio-genetics cardiologist.
"Lifestyle modification":
- All CPVT patients should avoid competitive and other strenuous exercise
Medication/Other therapies:
- β-blockers are first line therapy in CPVT because of their sympatholytical effect. However, β-blockers are not fully protective in all CPVT patients.
- Flecainide[4] can be given to CPVT patients with refractory ventricular ectopy despite β-blocker therapy. Flecainide directly blocks RyR2 channels.
- Left cardiac sympathetic denervation[5] can be done in selected patients and largely prevents norepinephrine release in the heart and may therefore reduce adrenergically mediated arrhythmias.
- The need for ICD therapy needs careful judgement since ICDs might not offer the ultimate protection in CPVT because of the fact that both appropriate and inappropriate shocks can trigger catecholamine release, resulting in arrhythmic ICD storms and death.
References
- Leenhardt A, Denjoy I, and Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012 Oct;5(5):1044-52. DOI:10.1161/CIRCEP.111.962027 |
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, and Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995 Mar 1;91(5):1512-9. DOI:10.1161/01.cir.91.5.1512 |
- van der Werf C, Nederend I, Hofman N, van Geloven N, Ebink C, Frohn-Mulder IM, Alings AM, Bosker HA, Bracke FA, van den Heuvel F, Waalewijn RA, Bikker H, van Tintelen JP, Bhuiyan ZA, van den Berg MP, and Wilde AA. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol. 2012 Aug 1;5(4):748-56. DOI:10.1161/CIRCEP.112.970517 |
- van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, Shimizu W, Sumitomo N, Fish FA, Bhuiyan ZA, Willems AR, van der Veen MJ, Watanabe H, Laborderie J, Haïssaguerre M, Knollmann BC, and Wilde AA. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011 May 31;57(22):2244-54. DOI:10.1016/j.jacc.2011.01.026 |
- Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, and Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008 May 8;358(19):2024-9. DOI:10.1056/NEJMoa0708006 |
- van der Werf C and Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: important messages from case reports. Europace. 2011 Jan;13(1):11-3. DOI:10.1093/europace/euq330 |
- Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, and DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002 Jul 2;106(1):69-74. DOI:10.1161/01.cir.0000020013.73106.d8 |